This article and associated images are based on a poster originally authored by Poppy Wood, James Craswell, Holly Madden, Alicia Galvan-Alvarez, Gabriel Negoita-Giras, David Gibson, Scarlett Turner, Andrew Ratcliffe, Phillip Fallon, and Nicholas Bland and presented at ELRIG Drug Discovery 2025 in affiliation with Domainex Ltd.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Background
Direct-to-Biology (D2B)
- Combines plate-based chemical synthesis with rapid high-throughput biological screening
- Removes lengthy purification steps by directly screening crude reaction mixtures for desirable activity
- Increases throughput and reduces cost compared to traditional synthesis
- Reduces the environmental impact of hit synthesis by limiting the need for solvent-heavy purification steps
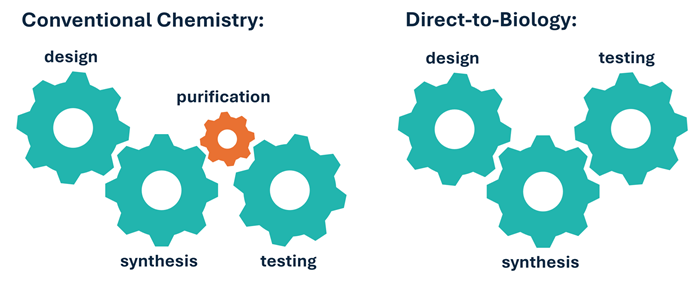
D2B removes the purification bottleneck from conventional chemistry, allowing for a flexible and efficient approach to hit finding. Image Credit: Image courtesy of Poppy Wood et al., in partnership with ELRIG (UK) Ltd.
Domainex’s PROTAC toolbox
- PROTACs are heterobifunctional molecules that dual-bind an E3 ligase and a protein of interest (POI) to induce POI degradation.
- Domainex has generated a proprietary PROTAC toolbox consisting of ~160 partial PROTACs (linker + E3 ligase binder).
- Toolbox includes Cereblon (CRBN) and VHL ligands, and a wide variety of linker lengths and types.
- Plate-based reactions of partial PROTACs with POI binders rapidly produce D2B plates with novel PROTAC candidates for screening.
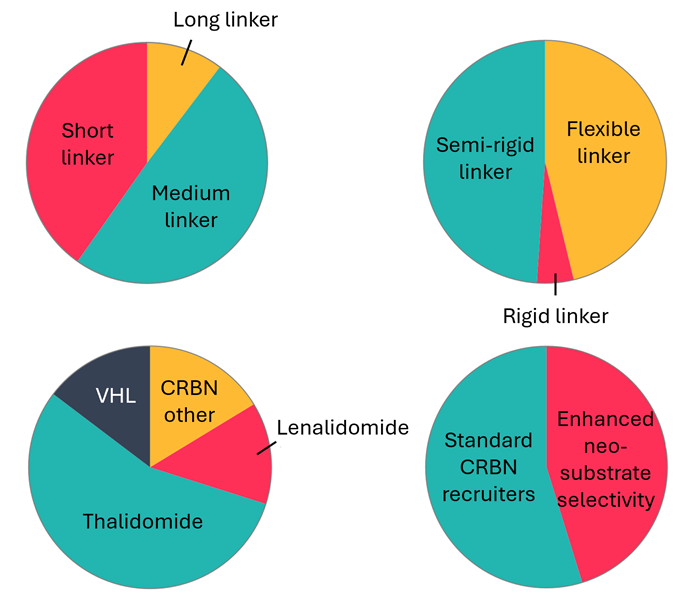
E3 ligase recruiter and linker compositions in Domainex's PROTAC toolbox. Image Credit: Image courtesy of Poppy Wood et al., in partnership with ELRIG (UK) Ltd.
Aurora Kinase A (AurA)
- Key cell cycle regulator (overexpressed in cancer)
- Literature precedence for CRBN-mediated AurA degrader
- Used as a tool target to demonstrate D2B potential for the development of CRBN-mediated novel PROTACs
AurA PDB ID: 4C3R. Image Credit: Image courtesy of Poppy Wood et al., in partnership with ELRIG (UK) Ltd.
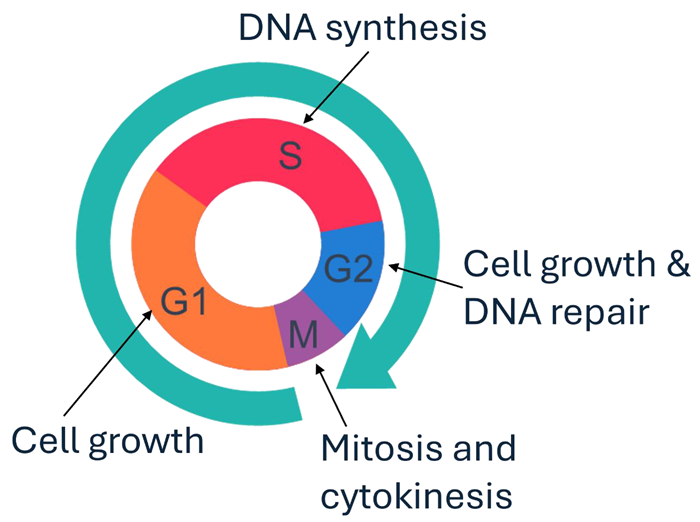
The cell cycle. Image Credit: Image courtesy of Poppy Wood et al., in partnership with ELRIG (UK) Ltd.
PROTAC® is a registered trademark of ArvinasOperations, Inc., and is used under license.
D2B-driven discovery of Aurora Kinase A degraders
PROTAC synthesis
- Linking Alisertib (AurA ligand) to Thalidomide (CRBN ligand) forms a potent and highly specific PROTAC-mediated AurA degrader (JB170) for use as a positive control.
- Plate-based chemistry was used to amide couple forty representative partial PROTACs to Alisertib for the generation of novel AurA degraders for screening.
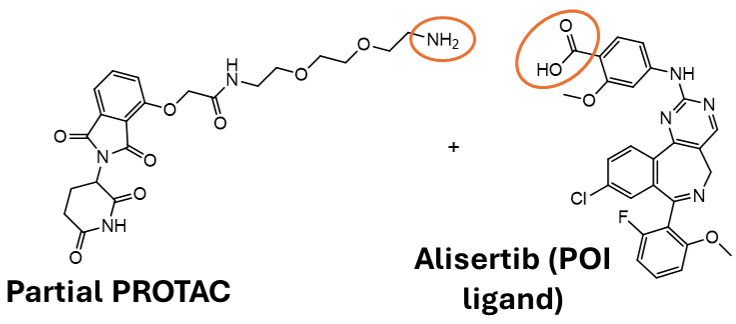
Amide coupling of Alisertib with partial PROTACs to form novel degraders. Image Credit: Image courtesy of Poppy Wood et al., in partnership with ELRIG (UK) Ltd.
Spectral shift: Driving a D2B campaign via biophysics
Spectral shift (NanoTemper) operates on the principle that ligand binding alters the chemical environment of a fluorophore, leading to measurable changes in the emission spectrum.
1. Single-shot binding screen
Crude D2B mixtures were screened at a single concentration against fluorescently labelled AurA to identify degraders capable of binding the target.
2. Ternary complex formation assay
Demonstrating ternary complex formation is a crucial step in any induced proximity modality, as seen here in the confirmation of PROTAC-mediated recruitment of CRBN to AurA.
Concentration-dependent binding profiles differ dramatically upon the inclusion of CRBN with AurA, indicating effective recruitment of the E3 ligase by the PROTACs.
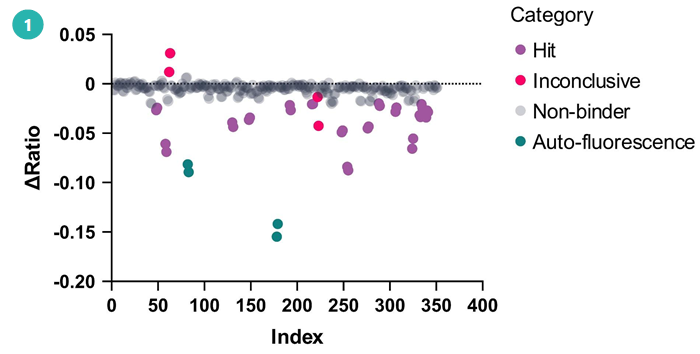
Single concentration binding assessment of D2B compounds to labelled Aurora Kinase A. Image Credit: Image courtesy of Poppy Wood et al., in partnership with ELRIG (UK) Ltd.
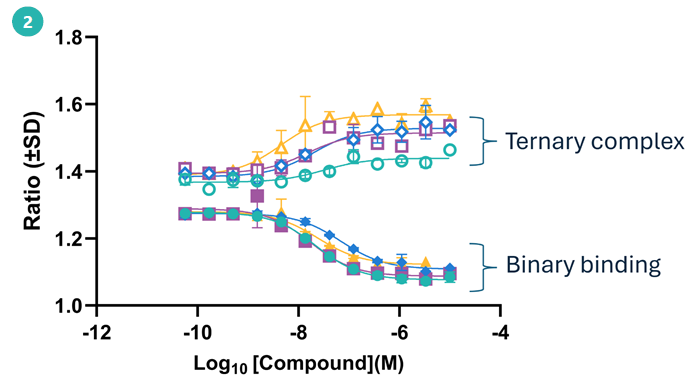
Concentration-dependent binary binding and ternary complex formation of select hits from single shot screen. Image Credit: Image courtesy of Poppy Wood et al., in partnership with ELRIG (UK) Ltd.
From spectrum to system: Cellular validation of D2B hits
HiBiT reporter assay
- HiBiT is an 11-amino acid tag that complements the LgBiT protein to form a functional luciferase, resulting in a luminescent signal proportional to the cellular HiBiT-tagged protein content.
- MOLT4 cells expressing HiBiT-tagged AurA were treated with JB170 and candidate novel PROTACs, resulting in a reduction in luminescent signal consistent with AurA degradation.
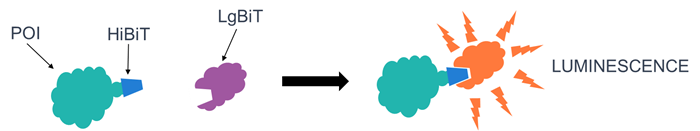
HiBiT assay system. Image Credit: Image courtesy of Poppy Wood, in partnership with ELRIG (UK) Ltd.
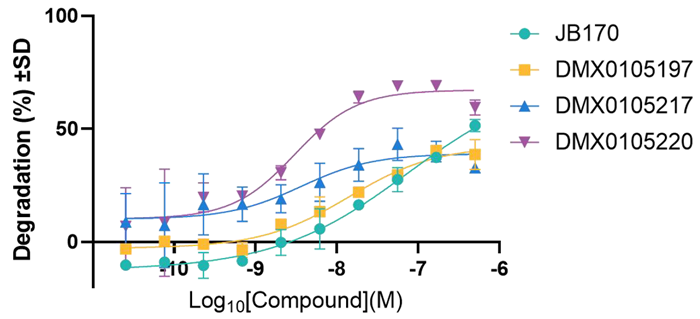
PROTAC-mediated concentration-dependent degradation of Aurora Kinase A by JB170 and novel PROTACs. Image Credit: Image courtesy of Poppy Wood et al., in partnership with ELRIG (UK) Ltd.
Using flow cytometry to measure cell cycle arrest
- THP1 cells were treated with concentration-response curves of JB170 and select D2B compounds.
- Following fixation, cells were stained with propidium iodide (fluorescent DNA intercalator) to measure DNA content.
- A cell cycle analysis model was applied to determine the proportion of cells in each phase of the cell cycle.
- Data shows JB170 and our novel PROTACs trigger cell cycle arrest in G2 phase in a concentration-dependent manner.
- This functional effect is consistent with AurA’s proposed role in regulating the transition from G2 to M phase of the cell cycle.
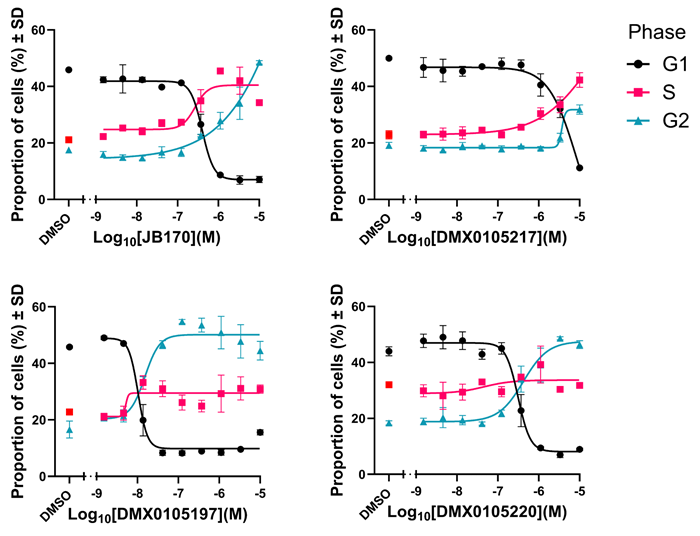
Compounds that induce degradation of Aurora Kinase A trigger concentration-dependent cell cycle arrest in the G2 phase. Image Credit: Image courtesy of Poppy Wood et al., in partnership with ELRIG (UK) Ltd.
Summary
Aurora Kinase A served as a model target for the integration of Domainex's PROTAC and D2B platforms, with both cellular and biophysical screening approaches used to rapidly identify novel degraders.
This approach could be applied to targeted protein degradation programmes and beyond, accelerating drug discovery to bring benefits to patients with unparalleled speed.
About Domainex
Domainex is a fully integrated drug discovery CRO based near Cambridge, UK. It serves pharmaceutical, biotechnology, academic and patient foundations globally. Domainex’s drug discovery service business was established in 2001 and since that time has continued to expand to serve a wider range of international clients including UCB, FORMA Therapeutics, St George’s University, and The Institute of Cancer Research.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free-of-charge to attend!
Our values
Our values are to always ensure the highest quality of content and that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world class conferences, networking events, webinars and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Nov 26, 2025