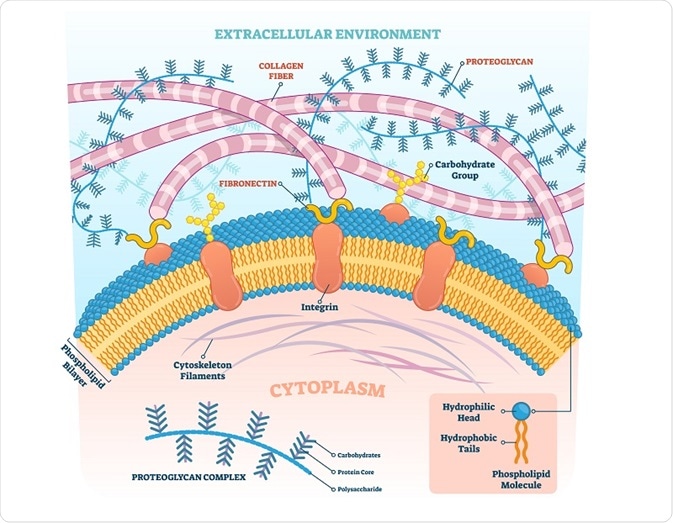
Proteoglycans are ubiquitous molecules that function as critical components of the extracellular matrix. These proteins are composed of glycosaminoglycan chains that are covalently attached to a protein core. Even though just a handful of proteins have the propensity to become proteoglycans, their range of functions and potential roles in organism development is extremely broad.
This article will cover:
 VectorMine | Shutterstock
VectorMine | Shutterstock
Structure of proteoglycans
Carbohydrate
Proteoglycans act as polysaccharides rather than proteins as 95% of their weight is composed of glycosaminoglycan. The glycosaminoglycan chains consist of alternating hexosamine and hexuronic acid or galactose units. There are also glycopeptide linkage regions that connect the polysaccharide chains to the core proteins that contain N- and/or O-linked oligosaccharides.
The regular polymer sequence of glycosaminoglycans is a result of the repetitive units of glycosaminoglycan chains. However, a study of glycosaminoglycan chains revealed marked heterogeneity in the individual polysaccharide units.
Core proteins
Proteoglycans can be classified on the basis of their core proteins. For example, one family consists of large extracellular chondroitin sulfate proteoglycans that can specifically interact with hyaluronic acid. Several proteoglycans in the extracellular matrix and proteoglycans present in different connective tissues belong to this category.
Another family of proteoglycan consists of small homologous core proteins that include one or two glycosaminoglycan chains. These small core proteins include decorin, biglycan, fibromodulin, etc. Heparan sulfate proteoglycans are present in the extracellular or basement membrane and are related to the heparan sulfate proteoglycan secreted by Engelbreth-Holm-Swarm (EHS) tumor cell line.
The serglycans are intracellular proteoglycans that consist of core protein sequences of serine and glycine units that are heavily substituted with heparan chains. All core proteins contain a glycosaminoglycan substitution domain and most of the proteoglycans are attached to macromolecules present in the extracellular matrix through domains that are present in the core proteins.
Glycosaminoglycan-substitution domains
The glycosaminoglycan chains are bound to the serine residues that are present in the core proteins. The elongation of the chain is initiated by xylosylation of specific serine residues. The serine units that are susceptible to xylosylation occur in the specific tetrapeptide sequence that is preceded by a few acidic residues. Synthetic peptides that contain this sequence have been shown to be adequate substrates for xylosylation in vitro.
Extracellular matrix | Structure of a cell | Biology | Khan Academy
Cellular functions
All cellular processes involve interactions at the surface of the cell, including the interaction of the cell with the matrix, with other cells, as well as with ligands. These interactions involve proteoglycans since these molecules bind avidly with the proteins and are abundantly present on the surface.
In one study, the role of heparan sulfate proteoglycans was described during the critical stages of development, including generation and differentiation of neurons, axonal guidance, synapse development, etc.
One of the critical behaviors of tumor cells is a local invasion and distant metastasis. These behaviors involve adhering to cells, motility, and growth - all of these factors are influenced by proteoglycans.
Another study showed that heparan sulfate proteoglycans can either inhibit or enhance these activities based on the cell or tissue type, pathophysiology of the tumor, and the specific step of metastasis that is affected.
Signal transduction
The two major families of cell surface heparan sulfate proteoglycans are syndecans and glypicans. These two families bind to several growth factors and matrix proteins and are involved in various signal transduction pathways implicated in the proliferation of cells and cell shape changes.
Syndecans are transmembrane proteins that are linked to the cell membrane using glycosylphosphatidylinositol (GPI) lipid anchors. There are four known mammalian syndecan proteins. While the structure of these proteins is quite similar to several shared cytoplasmic, juxtamembrane and transmembrane domains, they also have distinct regions and distributions inside cells. Both conserved and divergent protein partners have roles in the cellular and developmental functions of proteoglycans.
Recent studies conducted in Drosophila, mice, and humans showed how proteoglycans can be implicated in cell growth and differentiation. They also have particular roles to regulate the molecules involved in signaling pathways, such as FGFs, BMPs, Wnts, Hhs, IGFs, etc. However, the exact mechanism of how these molecules function is still not completely understood.
Functions in model organisms
Most of the information about how proteoglycans function comes from studies in the fruit fly and nematode worm, Caenorhabditis elegans. Many of the mammalian cell surface heparan sulfate proteoglycans have homologs in fly and worm.
While there are many differences in structure and function when fly and mammalian systems are compared, several functions of the Drosophila glypicans and syndecan are mimicked in mammals, suggesting conserved functions.
Further Reading
Last Updated: Jul 9, 2019