Research-backed strategy produces highly homogeneous antibody-drug conjugates (gsADCs) with consistent DAR and tumor suppression lasting over 50 days after a single dose
A new white paper from BioDlink, From Complexity to Consistency: How CDMOs Can Unlock One-Step, High-Yield and Homogeneous ADC Manufacturing for Next-Gen Oncology, reveals an advancement in antibody-drug conjugate (ADC) manufacturing - achieving over 95% conjugation yield in under one hour using a one-step glycoengineering strategy. The approach, based on research by Dr. Wei Huang et al., significantly reduces process complexity, manufacturing time, and analytical burden while enhancing product consistency and therapeutic performance.
With more than 210 ADCs in global clinical development, the field is reaching an inflection point where manufacturing limitations threaten to stall progress. Traditional conjugation processes involve multi-step reactions that span several days and often yield heterogeneous products with inconsistent drug-to-antibody ratios (DARs), typically ranging from 0 to 8. This variability increases regulatory uncertainty and risks delays in clinical advancement.
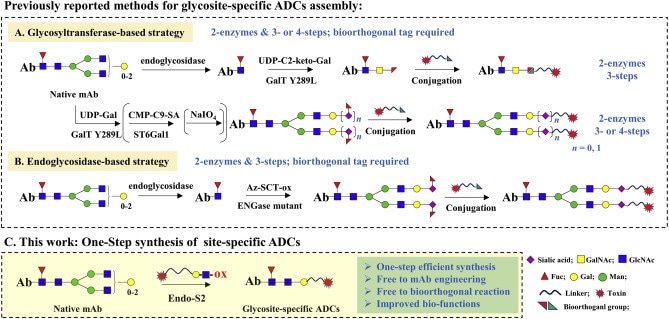
Figure 1. Strategies for synthesis of glycosite-specific ADCs
Key Findings:
High-Yield Conjugation in Record Time: The one-step LacNAc-based glycoengineering platform highlighted in this white paper enables over 95% conjugation yield in under one hour, drastically reducing manufacturing time compared to conventional multi-step ADC workflows.
Consistent Product Quality and Stability: The platform produces highly homogeneous gsADCs with a defined DAR of 2, verified through advanced analytics, and maintains low aggregation rates (10.2–14.6%) under stress conditions - supporting tighter CQA control and regulatory confidence.
Extended Therapeutic Response: In vivo studies show that a single dose of gsADCs delivers tumor suppression lasting over 50 days, outperforming heterogeneous ADC formats with higher DARs, offering stronger and longer efficacy in HER2-positive cancer models.
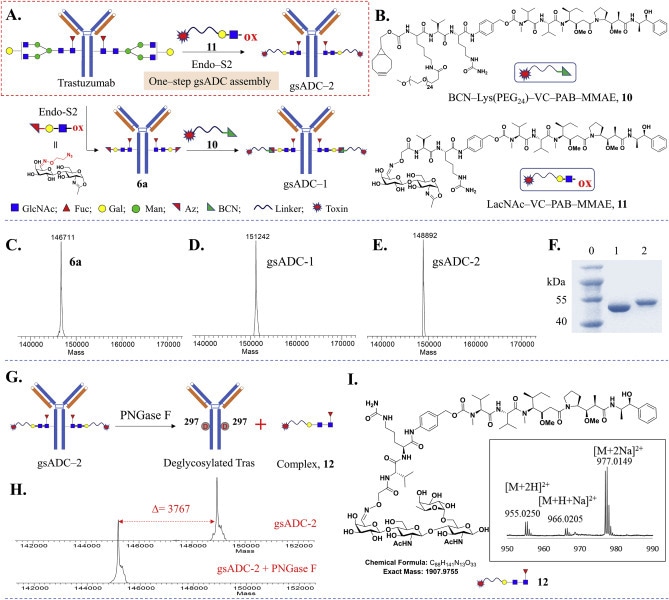
Figure 2. Synthesis of glycosite-specific ADCs using LacNAc-based substrates
As a leading global CDMO, BioDlink empowers biotech innovators through advanced technology platforms and strategic partnerships designed to accelerate and de-risk complex biologics development. Based on the core GL-DisacLink® platform technology developed by the Wei Huang team, BioDlink has achieved major breakthroughs in process scale-up, enabling high-yield, site-specific ADC conjugation in a single enzymatic step. Combined with its proprietary BDKcell™ cell line development system - capable of delivering stable clones in just 14 weeks - BioDlink offers a fully integrated solution that enhances product uniformity, reduces off-target effects, and streamlines workflows.
These innovations allow BioDlink to complete ADC development from DNA synthesis to toxicology-ready material in as little as 7 months and file full IND applications within 11 months. For monoclonal antibodies, the timelines are even faster at 6 and 10 months, respectively. In a globally competitive landscape, BioDlink focuses not on being the lowest-cost provider, but on delivering value through speed, quality, and reliability - building long-term, win-win partnerships that advance next-generation therapies to market.
Download the full white paper here: https://bit.ly/4qfLd4d