In the pharmaceutical sector, liposomes are often used as a drug delivery system to transport chemotherapeutic drugs to the tumor site. They are made up of phospholipids that have a polar end linked to a non-polar chain that arrange into bilayer vesicles, with the non-polar ends creating a bilayer and the polar ends facing the aqueous medium. In pharmaceutical practice, the active pharmaceutical ingredient (API) is usually inserted into the liposome, either by sandwiching it between the bilayers or by placing it in the hydophilic pocket, depending on its hydrophilicity (Figure 1).
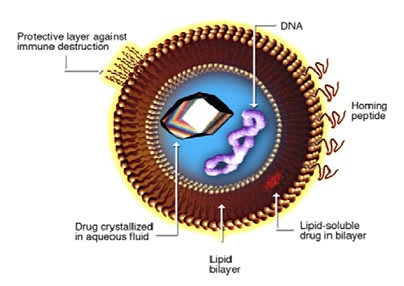
Figure 1. Liposome for drug delivery
DOXIL is a reformulated version of doxorubicin, one of the first drugs approved for delivery using a liposome. This drug is embedded within the hydrophilic pocket of a liposome coated with polyethylene glycol (PEG). This coating helps the liposome avoid being detected and destroyed by the immune system, thereby improving stability and extending the half life in circulation.
Liposomes can be divided into five main types based on their size, lamellarity, and preparation technique. These include:
- Small unilamellar vesicle (SUV)
- Small multilamellar vesicle (SMV)
- Multilamellar vesicle (MLV)
- Large unilamellar vesicle (LUV)
- Giant multilamellar vesicle (GMV)
Liposomes are also used in the creation of cosmetics such as creams and emulsions and in fields such as biotechnology for antibody delivery and siRNA delivery. The quantity of drug loaded into the liposome and the size of the liposomes are key to the drug’s pharmacodynamic and pharmacokinetic parameters. It is therefore necessary to ensure accurate and rapid measurement of liposome size for effective drug delivery.
The majority of liposomes measure around 20 to 250nm in size. Dynamic light scattering (DLS) is the preferred analytical technique for determining particle size. The Entegris Nicomp system is an example of a system used for this and is shown in Figure 2. There are some new GMV liposomes that are too large for DLS analysis, but these can be measured using the Entegris AccuSizer single particle optical sizing (SPOS) system (Figure 3).

Figure 2. The Nicomp N3000 DLS system

Figure 3. The AccuSizer SPOS system with SIS sampler
Size Measurements during Processing
The Entegris Nicomp system, together with the Entegris AccuSizer SPOS system are used to precisely determine the liposome size throughout manufacturing procedures such as extrusion through membranes. Figures 4, 5 and 6 show the size results obtained from the Nicomp DLS instrument while the liposomes are being extruded through membrane filters of reducing size.
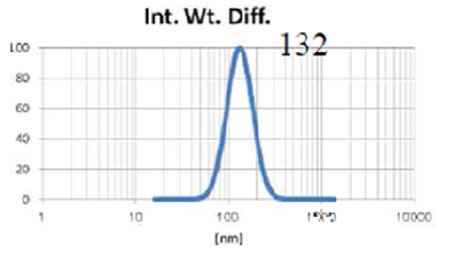
Figure 4. Liposome size before extrusion
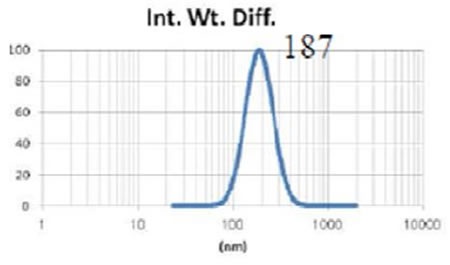
Figure 5. Liposome size after extrusion through 0.4 µm membrane three times
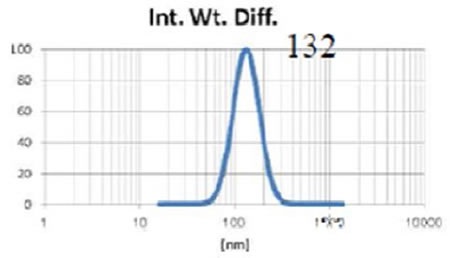
Figure 6. Liposome size after extrusion through 0.1 µm membrane three times
A sugar-doped, lipid-film hydration process was used to produce GMV liposomes. The size of the liposomes was then decreased through centrifugation and extrusion through membrane filters. The size changes were monitored using the AccuSizer SPOS system, as demonstrated by Figures 7 to 10.
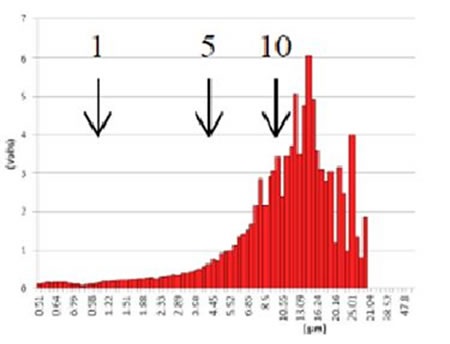
Figure 7. GMV liposome before centrifugation
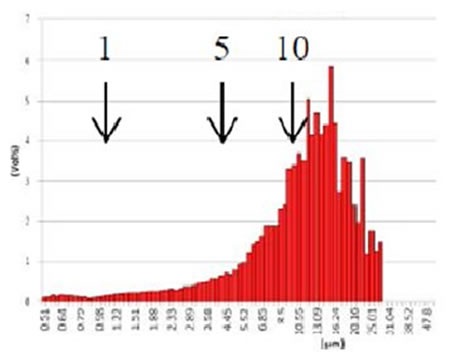
Figure 8. GMV liposome after centrifugation
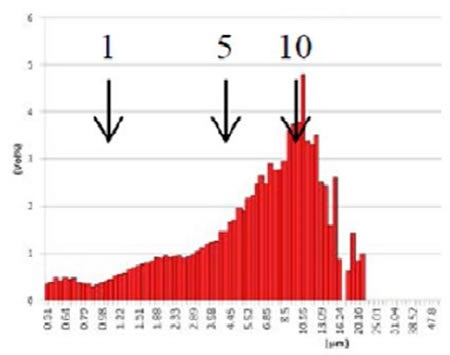
Figure 9. GMV liposome after extrusion through 5µm filter
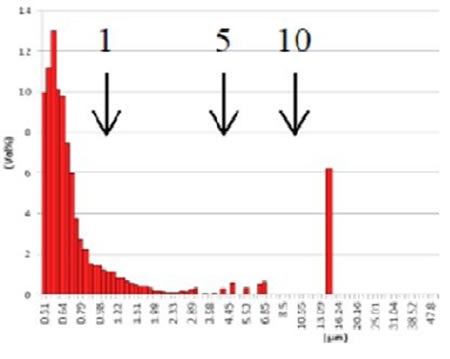
Figure 10. GMV liposome after extrusion through 10 µm filter Cationic Coated Liposomes
Cationic Coated Liposomes
For anionic DNA and RNA nucleotides, liposomes made up of the cationic lipid DOTAP (N-[1- (2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl-sulfate) serve as an effective carrier. Cationic liposomes offer several benefits, including high cellular uptake owing to the overall cationic electrostatic charge on lipid bilayers and high encapsulation efficiency of nucleotides. In order to prevent aggregation caused by serum, cationic liposomes have been coated with PEG to boost circulation lifetime and enable accumulation in tumor tissue.
At UC Davis, cationic liposomes were developed and analyzed that contained cationic lipid DOTAP for micro RNA encapsulation. Here, the liposome size plays a critical role, as these are eventually administered intravenously into mouse models. The end size of the liposomes should therefore be no more than around 100nm. Figure 11 shows the size result for cationic-coated liposomes.
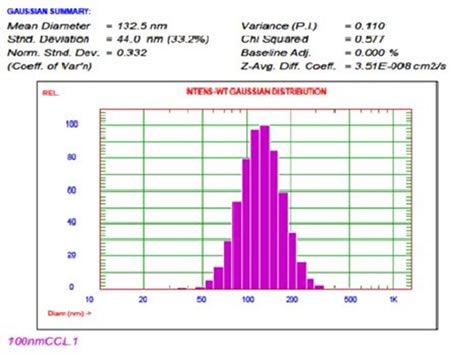
Figure 11. Cationic-coated liposome size result
Long Circulation Liposomes
Another liposome studied at UC Davis was labeled with 64Cu to serve as a nanotracer and enhance visualization of head and neck tumors using positron emission tomography or PET. This liposome is a specific formulation that is a combination of HSPC/cholesterol/DSPE-PEG2K, which gives a highly stable and long-circulating liposome (LCL) that is suitable for a wide range of applications. In this formulation, a molar ratio of 55.5:39:5.0 mol/mol/mol of HSPC/cholesterol/DSPEPEG2K was used and then functionalized with 6-BATPEG-lipid for 64Cu radio-labeling.
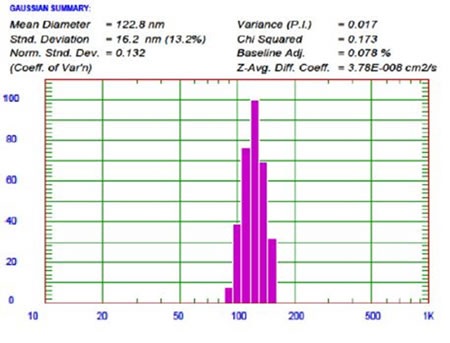
Figure 12. 64Cu labeled LCL liposome size result
Temperature Sensitive Liposomes
Liposomes that are sensitive to temperature have also been developed to improve the thermal-induced release of contents at a certain targeted area. In an experimental analysis, a pH-sensitive complex between copper (CuDox) and doxorubicin (Dox) was formed in the core of lysolipid-containing, temperature-sensitive liposomes (LTSLs). The liposomes were made up of DPPC:DSPE - PEG2k:MPPC at a molar ratio of 86:4:10, where MPPC is 1- palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine; DSPE-PEG2k is 1,2 distearoyl-sn-glycero-3-phosphoethanolamine-NMethoxy polyethyleneglycol-2000; and DPPC is 1,2- Dipalmitoyl-sn-glycero-3-phosphocholine.
Copper TEA liposomes were isolated from non-encapsulated copper TEA to promote a salt gradient across the liposomal membrane. Figure 13 shows the size of the MPPC-Copper TEA liposomes.
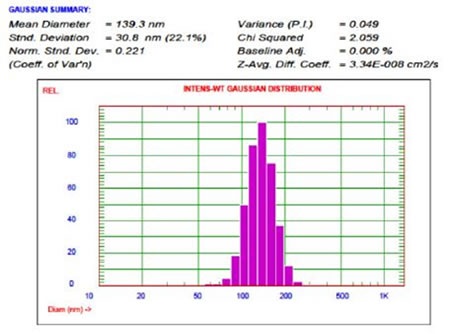
Figure 13. MPPC-Copper TEA liposomes.
Once the preparation of liposomes is complete and the size verified, the doxorubicin is loaded into the liposomes using the TEA gradient, where the drug enters the liposome as TEA comes out.
Zeta Potential of Zr-89 Labeled Liposomes
In another analysis, liposomes labeled with Zr-89 were produced to assess the pharmacokinetics of LCLs over seven days. The radioactivity is sequestered in the lipid bilayer, in the hydrophilic internal cavity, or on the liposome surface. The liposomes were measured on the Entegris Nicomp DLS system for size distribution, with values spanning from114 to 120nm. Using the dip cell and phase analysis light scattering mode (PALS), the zeta potential was also determined on the Nicomp DLS system. Measurement settings involved applying a 12V/cm electric field across a gap of 0.4cm between electrodes and using the Smoluchowski limit. Figure 14 shows the highly consistent results that were obtained from multiple measurements.
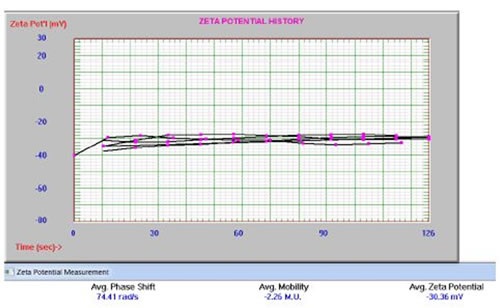
Zeta potential results for NH2-Peg2K liposomes
Conclusion
Liposomes are used in pharmaceutical, biotechnology and cosmetics industries. Important properties of these particles are size and surface charge, which need to be monitored and measured accurately. The DLS technique is widely used to determine the size of submicron liposomes. For liposomes greater than one micron, SPOS is used to determine their size. The Particle Sizing Systems’ Nicomp DLS system and AccuSizer SPOS system are used in laboratories across the globe for precise measurement of the charge (zeta potential) and size of liposomes.
References
- Aoki, N. & Hashimoto, M., Hashimoto Electronic Industry CO. and Yoshimura, T. Liposome Engineering Laboratory, “Measurement of Liposome Size Distribution Using Nicomp 380 and AccuSizer780 AD, presentation, July 2013
- Thanks to Elizabeth Ingham, Azadeh Kheirolomoom and Jai Seo from the Dr. Katherine Ferrara Lab in the Department of Biomedical Engineering at UC Davis for sharing these data and helping to create this document.
- Mahakian, L. et.al., Comparison of PET Imaging with 64Cu-Liposomes and 18F-FDG in the 7,12-Dimethylbenz[a] anthracene (DMBA)-Induced Hamster Buccal Pouch Model of Oral Dysplasia and Squamous Cell Carcinoma, Mol Imaging Biol (2013)
- Kheirolomoom, A. et.al., Complete regression of local cancer using temperature-sensitive liposomes combined with ultrasound-mediated hyperthermia, Journal of Controlled Release, 172 (2013).
- Seo, J. et.al., The pharmacokinetics of Zr-89 labeled liposomes over extended periods in a murine tumor model, Nuclear Medicine and Biology 42 (2015)
About Entegris
For over 35 years Entegris has been committed to helping customers find solutions to their particle sizing problems. We offer products with unique capabilities that can size particles from single digit nanoparticles to particles that are thousands of microns in diameter. Whether you need a particle size distribution or to find that needle in a hay stack, our team of engineers and sales staff are available to provide the product knowledge and scientific expertise necessary to solve your sizing issues.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and iform site visitors interested in medical research, science, medical devices and treatments.