Emulsions are two-phase systems consisting of a dispersed phase (the droplets or particles) and a continuous phase (liquid diluent like water). In the dispersed phase, droplet size is critically important. Most droplet size measurements are made in the laboratory. However, these measurements can now be measured continuously utilizing the Entegris Mini dynamic light scattering (DLS) system.
There are numerous types of emulsions – such as oil in water (OIW), water in oil (WIO), and complex double emulsions. Most pharmaceutical emulsions are oil-in-water emulsions where the active ingredient is in the oil phase and the dispersed phase is water.
The product is typically called an emulsion if the drug delivery route is internal (injected or swallowed). If the drug delivery route is topical (through a patch or spread on the skin), the product is usually called a lotion or cream.
The droplet size is vital for stability and quality purposes,1 and can occasionally be classified into three ranges:2
Source: Entegris, Inc.
| Type |
Size Range |
| Macroemulsions |
0.1 – 100 μm |
| Microemulsions |
5 – 50 nm |
| Nanoemulsions |
<100 nm |
Processes
A variety of process equipment is available for producing emulsions and suspensions at both the laboratory and commercial scale. This includes turbine mixers, homogenizers, propeller mixers, colloid mills, ultrasonicators, and microfluidizers. Figure 1 shows a basic process flow chart for creating a lipid emulsion.
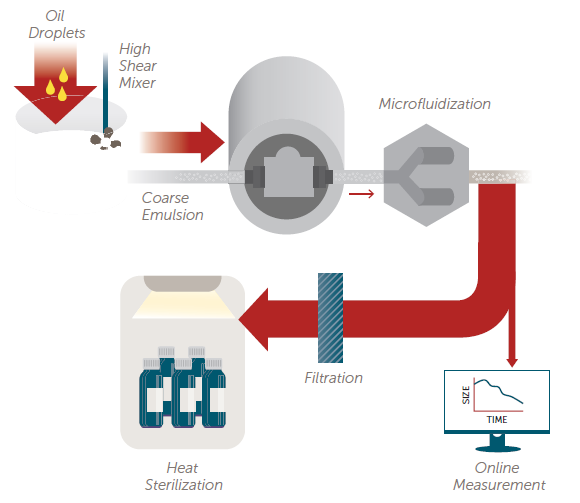
Figure 1. Emulsion process flow chart. Image Credit: Entegris, Inc.
DLS
Laboratory DLS instruments are the most common analytical technique utilized to measure the droplet size of micro- and nanoemulsions. Testing the droplet size of lipid injectable emulsions is explained in detail in USP 729.4,5
Laser diffraction, or DLS, is utilized to measure the mean size (Method I). Single particle optical sizing (SPOS) is utilized to measure the tail (Method II) of the distribution larger than 5 μm.
To pass the Method I specification, the mean size must be under 500 nm. Currently, these tests are executed on lab systems like the Entegris Nicomp system. Using the Entegris Mini DLS system, these measurements can now be performed continuously in-process.
Mini DLS system
The Mini DLS is an elegant and flexible solution. It can be modified for various nanoparticle manufacturing processes – such as homogenizing, milling, or microfluidizers.
A pressurized stream of suspension product connects to the Mini DLS system. This sample is then automatically diluted to attain a suitable light scattering intensity for the measurement (Figure 2).
The particle size distribution is calculated, and the system is automatically flushed and cleaned. The measurement sequence is then subsequently repeated.
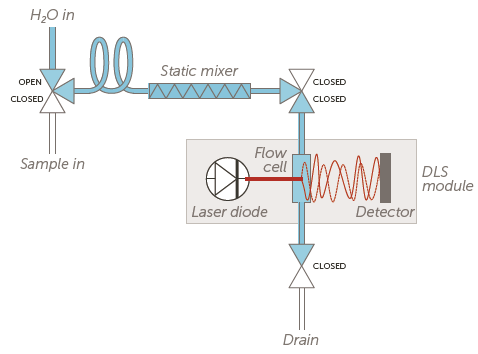
Figure 2. Mini DLS system flow chart. Image Credit: Entegris, Inc.
The advantages of utilizing the Mini DLS include:
- Automated sampling and measurement to remove laboratory and operator resources.
- Adaptable protocols to enhance measurement to application.
- Continuous data to monitor fluctuations in particle size distribution.
- Simple data transfer to process monitoring control software.
The operating screen for the Mini DLS offers a graphical user interface to track the system operation – as shown in Figure 3.
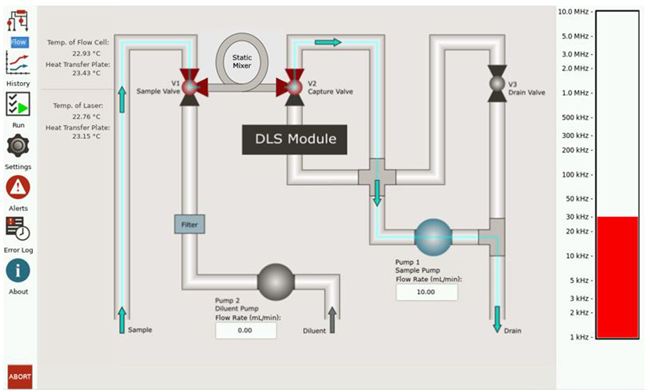
Figure 3. Mini DLS control screen. Image Credit: Entegris, Inc.
The left sidebar features icons for selecting the desired display view. In the center, a flow diagram presents the dilution fluidics and the current operating conditions. On the right, a bar graph displays the current light scattering level, measured in kHz.
A typical result for the Mini DLS system is shown in Figure 4.
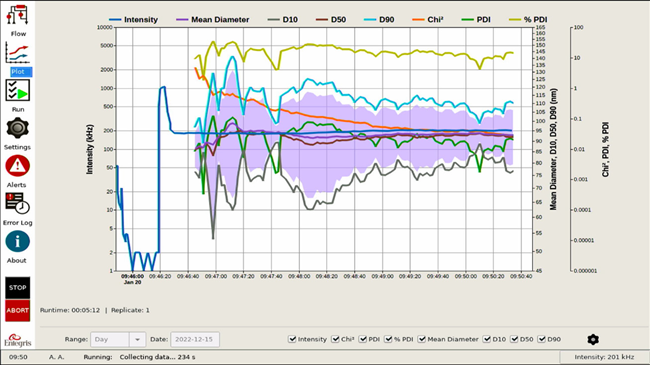
Figure 4. Size vs. time plot. Image Credit: Entegris, Inc.
The result values plotted vs time include:
Source: Entegris, Inc.
| . |
. |
| Intensity |
light scattering in kHz |
| Mean diameter |
nanometers |
| D10, D50, D90 |
key sizes in nanometers |
| Chi2 |
goodness of fit parameter (dimensionless) |
| PDI |
width of distribution (dimensionless) |
| %PDI |
width of distribution as a percent |
Experimental – simulated inline measurements
Pharmaceutical emulsions are the most common samples analyzed by the Mini DLS system. The results highlighted here are emulsions sampled from a beaker that contains the pre-diluted emulsion.
Sample pump #1 draws the sample from the beaker – as shown in Figure 3. Sample pump #1 is not utilized for in-process pressurized lines.
Intralipid is a synthetic fat emulsion made of egg phospholipids, glycerin, soybean oil glycerol, and water. It is a source of essential fatty acids and calories and is thus utilized to provide calories to patients who receive their nutrition through intravenous injection.
Two measurements on the Mini DLS system of an older 10 % Intralipid sample are shown in Figure 5. In between the sample results is a white space that represents the cleanup time of two minutes.
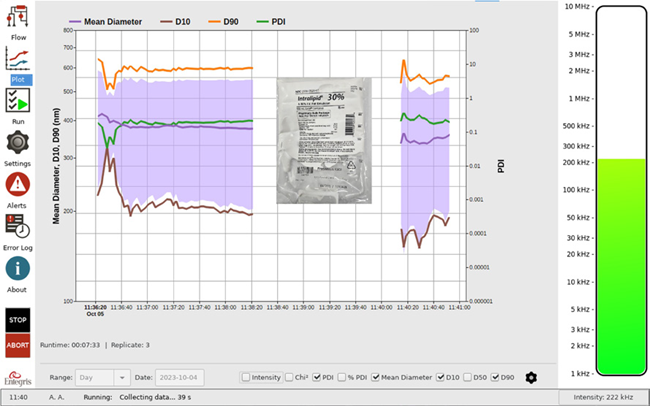
Figure 5. Intralipid size and PDI vs. time. Image Credit: Entegris, Inc.
The intensity mean size should be below 500 nm; in these results, it lies between 360 nm and 380 nm. The PDI values average just above 0.12. Due to the age of the sample, the PDI values and the intensity mean could be more than expected in a new batch.
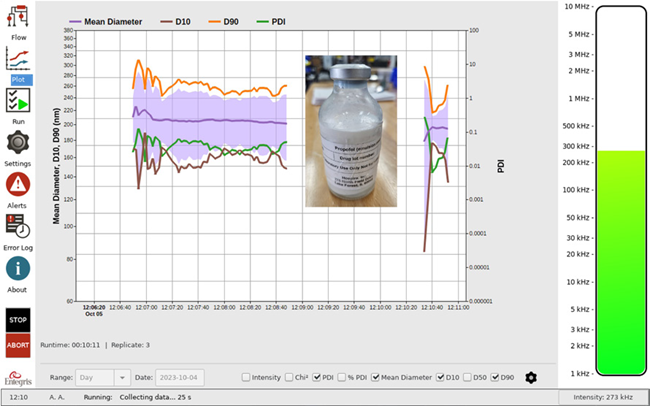
Figure 6. Propofol size and PDI vs. time. Image Credit: Entegris, Inc.
Propofol is a short-acting medication that decreases consciousness and causes a lack of memory for events. It is utilized for sedation and general anesthesia. The intensity mean size should be between 150 nm and 300 nm.
Figure 6 shows the results collected on the Mini DLS system. The intensity mean results are between 190 nm and 220 nm, with a PDI value of > 1. Again, the PDI value may be heightened because of the sample’s age.
The intensity mean continues to decrease during the two-minute measurement, implying that a longer analysis time would be suggested (because of the polydispersity of the sample). For samples with PDI > 1, the standard analysis time would be five minutes at least.
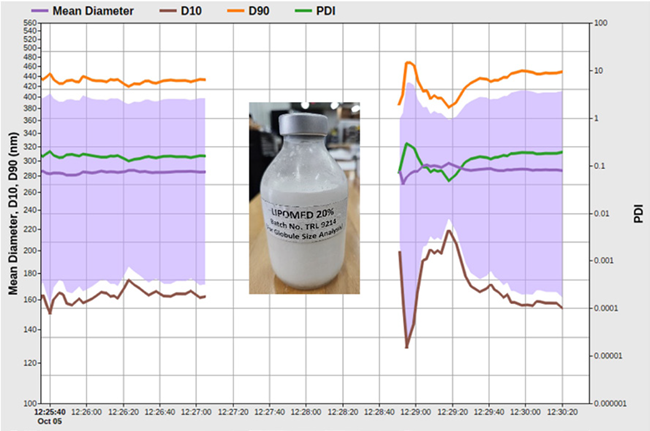
Figure 7. Lipomed size and PDI vs. time. Image Credit: Entegris, Inc.
Lipomed 20 % is administered as part of a total parenteral nutrition (TPN) regimen. The sample is made of medium-chain triglycerides, soya oil, tocopherol, egg lecithin, sodium hydroxide, sodium oleate, and water for injection.
The mean size and PDI versus time results for an older Lipomed sample are shown in Figure 8. The intensity mean size averages at approximately 290 nm; the PDI value is just below 0.1.
Experimental – in process
The Entegris inline DLS system was installed downstream of a high-pressure homogenizer. This setup enables it to grab an emulsion sample from the process stream approximately every 2 minutes.
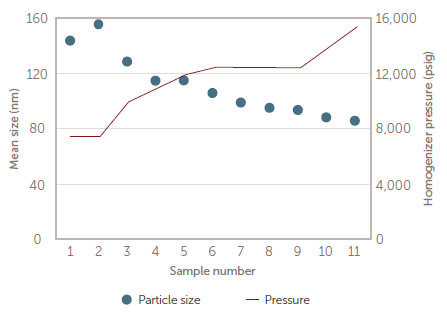
Figure 8. Size vs. pressure response. Image Credit: Entegris, Inc.
The emulsion sample is diluted in a buffer like the process fluid and auto-diluted to a concentration that creates an ideal light scattering intensity. A preliminary experiment determined the relationship between produced droplet size and homogenizer pressure – the response of size-to-pressure is approximately 9 nm per 1000 psig (see Figure 8).
A product batch was subsequently produced under slightly varied process conditions, resulting in the first two in-process samples being smaller than target size. After correcting the pressure, the size was returned to target for the last four samples – as shown in Figure 9.
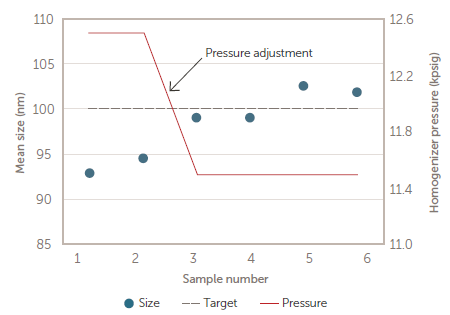
Figure 9. Size vs. pressure for production batch. Image Credit: Entegris, Inc.
Conclusion
Emulsion droplet/particle size can be continuously examined using the Mini DLS system, delivering process control and guaranteeing product quality. The touchscreen and easy user interface make generating protocols and conducting measurements simple.
Results are accessible as a function of time, or in-depth information can be shown for distinct measurements. Results can be transferred in JSON standard file format into data acquisition systems.
References
- Entegris application note, “Emulsion Stability”, https://www.entegris.com/content/dam/shared-product-assets/sensing-and-control/appnote-emulsion-stability-10544.pdf
- McClements, D.J., “Nanoemulsions versus microemulsions: terminology, differences, and similarities”, Soft Matter, 2012, 8, 1719–1729 | 1719
- Entegris application note, “Size Reduction by a Microfluidizer”, https://www.entegris.com/content/dam/product-assets/nicompnanodlszlssystems/appnote-size-reduction-by-microfluidizer-10541.pdf
- USP <729> “Globule Size Distribution in Lipid Injectable Emulsions”
- Entegris application note, “Globule Size Distribution in Lipid Injectable Emulsions”, https://www.entegris.com/content/dam/product-assets/accusizerspossystems/appnote-usp-729-globule-size-distribution-lipid-injectable-emulsions-10537.pdf
About Entegris
For over 35 years, Entegris has been committed to helping customers find solutions to their particle sizing problems. We offer products with unique capabilities that can size particles from single digit nanoparticles to particles that are thousands of microns in diameter. Whether you need a particle size distribution or to find that needle in a hay stack, our team of engineers and sales staff are available to provide the product knowledge and scientific expertise necessary to solve your sizing issues.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and iform site visitors interested in medical research, science, medical devices and treatments.