Reviewed by Alex SmithMay 9 2022
Proteins are the fundamental building blocks of life, and they are the essential products of molecular biology’s core dogma. Proteins play a variety of roles in the body, including catalyzing metabolic events, creating cellular matrices, and building signaling pathways to respond to external stimuli.
The study of protein structure and function is currently being conducted by scientists from all fields because they hold the key to understanding the “meaning of life.”
Due to the difficulty of obtaining most proteins of interest, it is critical to identify sources that might give researchers a limitless supply of proteins. The technique of cloning a gene encoding a protein-of-interest into an expression vector (typically a plasmid) and transferring it into a host cell for protein production by leveraging the host cell’s inherent protein synthesis machinery is known as recombinant protein expression.
For recombinant protein manufacturing, several host cell systems have been devised, and choosing the best host for any particular protein is a key aspect of its effective expression. Table 1 summarizes the benefits and drawbacks of frequently used expression hosts.
Table 1. Commonly used host cells for recombinant protein expression. Source: Sino Biological Inc.
| Expression Host |
E.coli |
Yeast |
Insect |
Mammalian |
| Category |
Prokaryote |
Eukaryote |
Eukaryote |
Eukaryote |
| Culture density |
High |
High |
High |
Medium |
| Culture duration Short |
(1~2 days) |
Short (1~2 days) |
Medium (2~4 days) |
Long (5~7 days) |
| Protein folding |
Limited |
Yes |
Yes |
Yes |
| PTM |
None |
Glycosylation |
Glycosylation, phosphorylation… |
Glycosylation, phosphorylation… |
| Suitable proteins |
Proteins with low MW |
Secreted, intracellular |
Secreted, intracellular |
Secreted |
| Cost |
Low |
Low |
High |
High |
| Example Cell Lines |
BL21(DE3), Rosetta… |
pichia pastoris |
sf9, sf21, High-five |
HEK293, CHO |
| Note |
Inclusion bodies |
High mannose glycan |
Low MW glycan |
|
Baculovirus-insect cell system (BEVS)
Insect cells require baculovirus as an intermediate to transfer a target gene into the cells and achieve protein production. Baculoviruses are a varied collection of DNA viruses capable of infecting a wide range of insect cells (>600). They act as a vehicle for delivering a target gene to a specific host cell.
The best-characterized baculovirus for this purpose is Autographa californica multiple nucleopolyhedrovirus (AcMNPV), which is commonly employed for insect cell-mediated protein production. Figure 1 shows a flow chart of this procedure. In brief, a gene encoding the desired protein is introduced into a primary vector, which is then cloned into a secondary vector known as Bacmid.
Bacmid is introduced into a bacterium strain (usually E.coli) for viral generation and assembly to get generation 1 baculovirus (P1). The P1 virus is amplified in an insect cell (e.g., sf9) to an appropriate titer (P2) before being utilized to infect the same or various insect cell lines (e.g., High-five) for protein expression.
This “Bac-to-Bac” system has been modified to produce a wide range of proteins as secreted, intracellular or membrane-bound molecules. There are several commercially accessible baculovirus generation techniques.
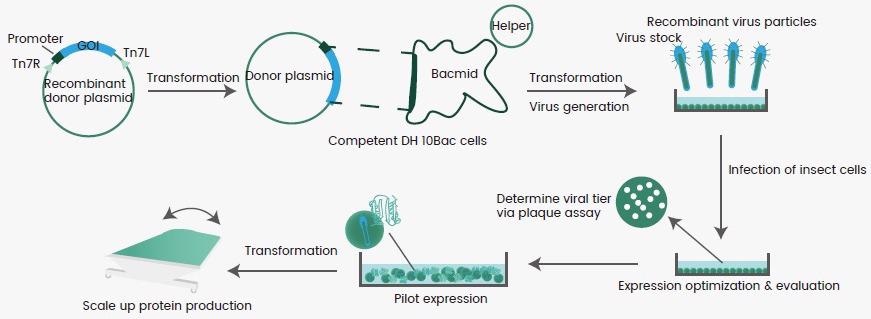
Figure 1. Flowchart for recombinant protein expression in insect cells. Image Credit: Sino Biological Inc.
Application of BEVS
Insect cells may be used to express a wide variety of recombinant proteins. Due to their powerful protein folding capabilities and relatively high culture density, they are great alternatives for the production of complex intracellular and viral proteins. Cervarix, a human papillomavirus vaccine made from insect cell lines and packaged as virus-like particles, was authorized for human use in 2007.
Structure elucidation, medication design, assay creation, and diagnostic reagent development are all areas where highly active proteins generated in insect cells are exploited. From construct design and culture optimization through protein purification and formulation, a systematic approach is necessary to create fully functioning recombinant proteins.
Researchers present two examples to show how BEVS may be used to highlight fundamental properties of recombinant protein production.
Core region fusion
Due to their better folding and posttranslational modification capabilities, insect cells are frequently utilized to create big molecular weight (MW) proteins (MW > 150 kDa). However, one major drawback of this is the structural complexity of such proteins, which generally results in low yields (Figure 2, left).
An alternative approach is to express a domain of interest rather than the full protein; however, due to low protein production and high degradation, direct expression of two enzyme domains of human fatty acid synthase (FASN) was not viable in this circumstance (Figure 2, middle).
Experts successfully developed a high-yield construct by extrapolating and fusing the sequences encoding the methyltransferase and ketoreductase domains with a linker (Figure 2, right). This construct’s Elute 1 and 2 were combined and purified to produce a final fusion protein with >90% purity.
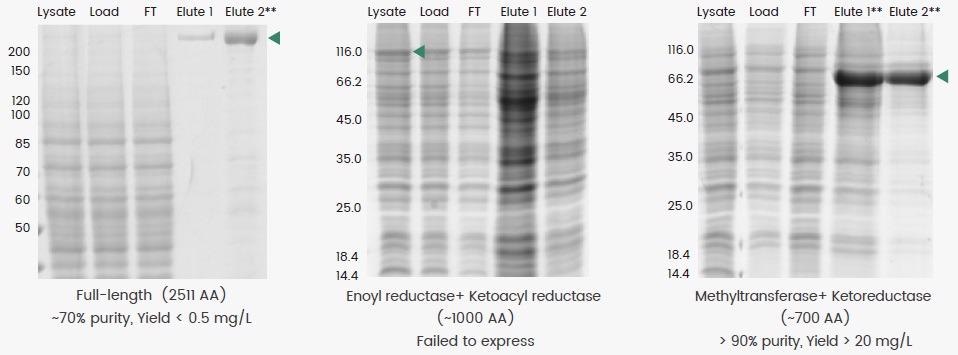
Figure 2. Expression of full-length (left), truncated (middle), and methyltransferase plus ketoreductase domain fusion (right) constructs of human FASN. The full-length protein was prepared as reported by Hardwicke et al. (2014, doi:10.1038/nchembio.1603), whereas the domain fusion construct was prepared as reported by Lu et al. (2018, doi: 10.1016/j.bmcl.2018.05.014). Image Credit: Sino Biological Inc.
Obtaining protein with the correct oligomeric status
To be functional, some proteins require certain oligomeric forms. For example, influenza virus hemagglutinin proteins and SARS-CoV-2 spike proteins are trimeric, but FAP, the human prolyl endopeptidase, is an inherent dimer. To guarantee adequate protein activity, caution should be used during the purification procedures to monitor the protein fractions with the right oligomeric condition.
BEVS was used to produce a his-tagged protein, and the Ni-affinity eluate included a combination of monomer and dimer. A second gel-filtration purification process was necessary to enhance the protein dimer since it is active as a dimer.
The end product’s dimeric structure was validated by SEC-HPLC, and the protein’s enzymatic activity was consistent across two batches utilizing the established method.
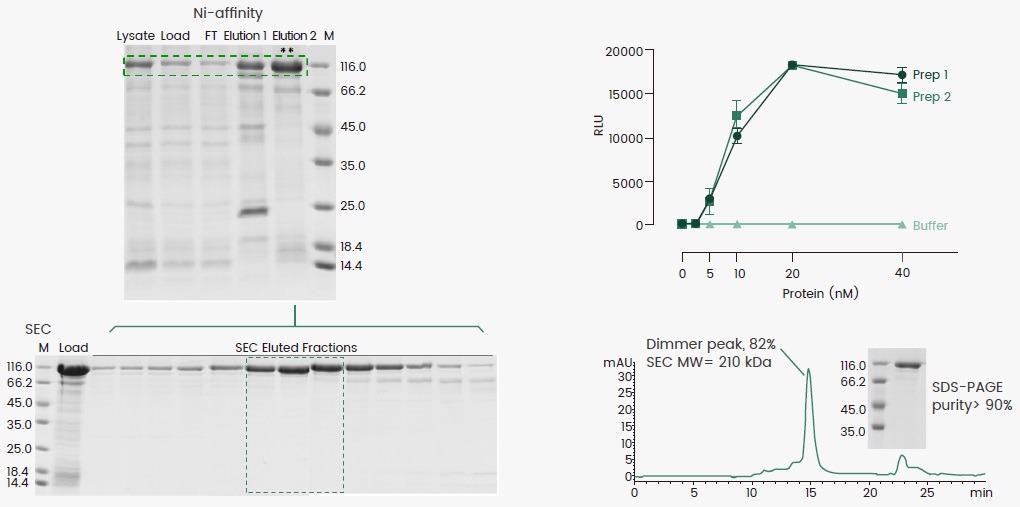
Figure 3. Expression and purification of an active protein dimer. The Ni-affinity Eluate 2 was subjected to gel-filtration chromatography to enrich the protein dimer. The dimeric status of the protein was assessed by SEC and the activity was determined using the corresponding enzymatic assay. Image Credit: Sino Biological Inc.
Conclusive remarks
Recombinant proteins are essential to the development and study of biologics. Insect cells are the best choice for expression hosts because they allow for proper protein folding and posttranslational modification, as well as being ideal for high-density cell culture. They can make diverse species’ secretory and intracellular proteins. To create high-quality recombinant proteins utilizing insect cells, a systematic design and optimization method are required.
About Sino Biological Inc.

Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.