The applications of mass spectrometry (MS) in pharma have long been established. Mass spectrometers are highly versatile and are a popular instrument for the identification of contaminants, reagents, products, and impurities from samples in the research and development, scale-up, and high-volume manufacturing lines of drugs.
MS instruments are generally substantial in size, as well as being power-hungry and costly. They are also typically operated in centralized laboratories by MS specialists.
The new requirements of bioprocessing to make biologics have resulted in a need for analytical instruments, such as mass spectrometers, to offer real-time information (online and at-line) at the point of need.
MS delivers information-rich data which can aid in addressing the growing complexities of bioprocessing.
This article outlines how Microsaic System’s compact and deployable mass spectrometer can be used to sample media from a bioreactor in real-time to deliver important process monitoring and critical quality attributes of the product.
This information can then be used to maximize yields of biologics by optimizing the cell media, feeding, and harvesting strategies of the target biologic being produced.
MS point-of-need analysis also offers timely safety assurance since any harmful PTMs of the product, or other dangerous host cell proteins, are able to be controlled and minimized upstream.
Figure 1. Prototype mass spectrometer based on the Microsaic 4000 MiD ® and MiDas sampling. Image Credit: Microsaic Systems
Experiment
A prototype system was utilized based on the Microsaic 4000 MiD® mass spectrometer and the MiDas sampling interface unit, as presented in Figure 1. The mass spectrometer offers an adjustable mass range between 50 and 3200 m/z, enabling the accurate identification of both proteins and small molecules.
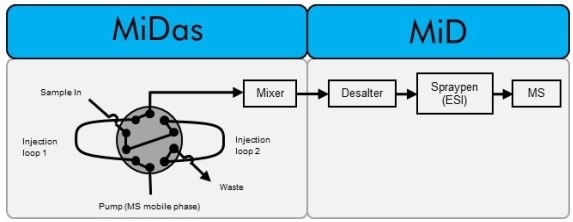
Figure 2. Experimental setup showing the various steps and functions of the MiDas and MiD to analyze cell media and proteins. Image Credit: Microsaic Systems
The prototype interface unit facilitates the sampling, preparation, and separation of biological liquids from bioreactors into an easily ionizable electrospray and mass spectrometer-compatible mobile phase.
Details of the experimental set-up are presented in Figure 2, while representative workflows of both biologic CQAs and cell media monitoring are presented in Figures 3 and 4.
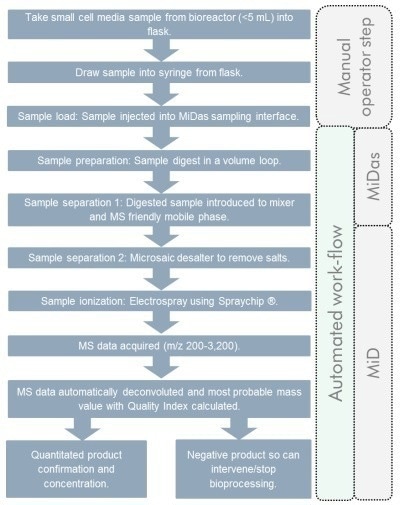
Figure 3. Work-flow of biologic products monitoring in upstream bioprocessing. Image Credit: Microsaic Systems
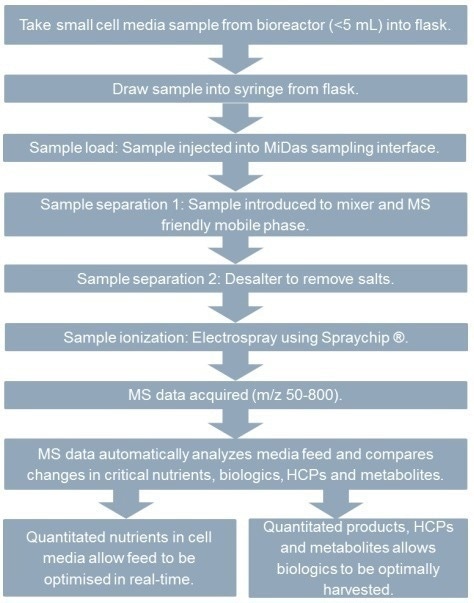
Figure 4. Work-flow of cell media monitoring in upstream bioprocessing. Image Credit: Microsaic Systems
Results and discussion
Representative proteins were sampled and tested with an identical set-up for cell media testing. Figure 5 displays the mass spectra of various proteins where important CQAs may be easily acquired using a real-time analysis tool, such as the automatic protein mass finder which is also shown in Figure 5.
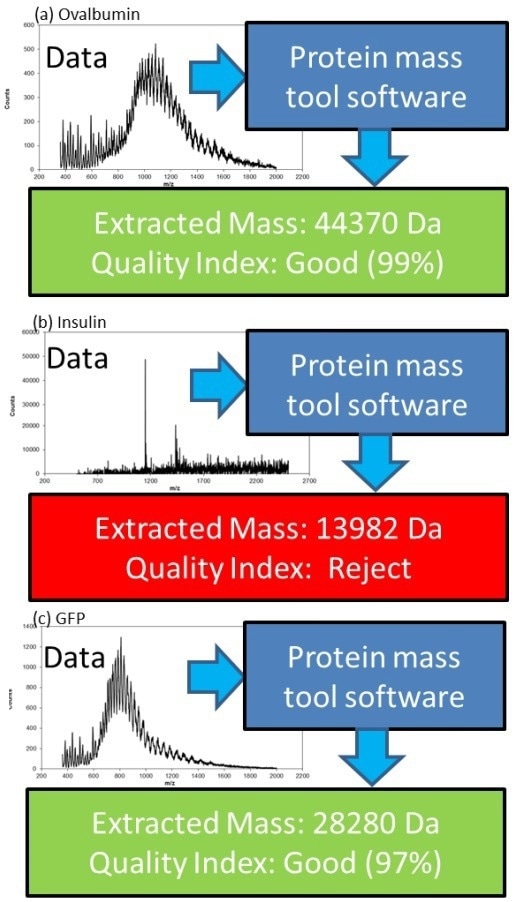
Figure 5. At-line biologic product CQA monitoring in upstream bioprocessing (a) Ovalbumin (b) Insulin and (c) GFP his-tagged. Image Credit: Microsaic Systems
This tool converts the information-rich data provided by the MS into data that is easy to understand, comprising the most prominent protein mass as well as a ‘Quality Index’ for each mass.
The ‘Quality Index’ offers a simple-to-understand certainty of a protein’s mass, in addition to the input data’s quality.
This data reduction allows data from the MS to be easily understood and allows the operation of the MS close to the point of need by the non-expert.
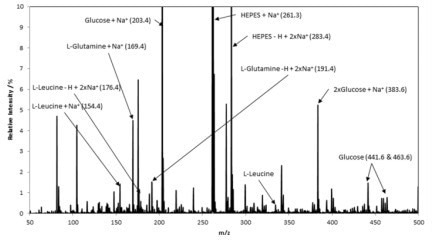
Figure 6. At-line biologic product CQA monitoring in upstream. Image Credit: Microsaic Systems
A representative sample of cell media was sampled and then analyzed. A typical mass spectrum is displayed in Figure 6, which identifies five components of the media mix. Following this, the chemical information can be utilized for controlling the bioprocess through optimization of the media, feeding, and harvesting strategies.
Conclusion
The experiment detailed in this article demonstrates the potential of Microsaic’s deployable MS for real-time point-of-need applications in upstream bioprocessing, including cell media makeup optimization, cell media monitoring and feeding, and biologic product CQA monitoring.
Microsaic’s portfolio provides the biopharmaceutical industry with a valuable tool to produce safer and cheaper biologics.
Contact Microsaic Systems to discuss your specific requirements.
About Microsaic Systems
Founded in 2001, Microsaic Systems plc (AIM: MSYS) develops real-time, point-of-need mass spectrometers. Microsaic offers fast, accurate cutting-edge solutions to multiple industries across the world.
Core products, such as the compact MiD series of mass detectors, are designed to integrate seamlessly with a wide range of third-party OEM equipment or used as a standalone system. At the forefront of our design ethos is to deliver fast, easy to use, powerful mass spectrometry (MS) performance.
Patented chip-based MS technology and intuitive software enables real-time data generation at the point-of-need, not just in a centralised laboratory. Designed for both pharmaceutical and biopharmaceutical applications, continuous data is accessible at any stage of your workflow.
Over 20 years’ experience in mass spectrometry, microfluidics, vacuum systems, analytical processes, and miniaturised instrumentation has led to the development of our outsourced services. Laboratory, engineering, and monitoring issues can now be solved with a world-class team of chemists, physicists, and engineers by your side.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.