Sponsored Content by InProcess-LSPReviewed by Louis CastelJun 19 2025
There are a number of techniques currently used by the pharmaceutical industry for particle size determination. However, most of these are applicable in a visible particle range (> 25 μm) and are low-throughput methods. Conventional DLS used for nanoparticle analysis requires sample handling (concentration and turbidity optimization) and cannot be done in a non-destructive manner (container integrity).
Spatially Resolved Dynamic Light Scattering offers unique capabilities to measure non-destructively through various packaging materials without the need for any sample handling. This report showcases the potential of SR-DLS, the NanoFlowSizer, for particle analysis inside closed containers of arbitrary geometry, allowing time-efficient and user-friendly analysis of all types of (complex) samples (sterile, toxic, light sensitive, degradable). Sample analysis can be performed off-line (QC laboratory), at line (manufacturing location), or in-line (continuous, real-time measurement).
Introduction
Nanoparticle size is a Critical Quality Attribute (CQA) for many products and formulations, and thus, it requires reliable and robust measurement protocols. These protocols typically involve the development of the analytical method of sampling: representative extraction, controlled preparation, dilution protocols, transport to characterization facilities, and training specialists.
Smooth transfer from lab to manufacturing requires an elaborate experimental setup and data evaluation to ensure adequate control of key process parameters. Analytical strategy throughout the process development and later manufacturing is critical for successfully implementing manufacturing processes and later product release.
Dynamic light scattering (DLS) is the widely adopted technique for measuring particle size (range 1 nm – few µm) in colloidal suspensions. The measurements are typically done in a cuvette containing the (diluted) sample by passing a laser beam through the sample without physical contact between the probes and the sample. This technique can only be used destructively, as the container integrity needs to be compromised to perform the measurement.
The next step in nanoparticle measurement is the ability to measure particle size inside closed containers of any geometry, at the product’s native turbidity, and in real time. This eliminates the need for sample handling and removes the risk of compromising product sterility or exposing its contents to the environment.
Spatially Resolved Dynamic Light Scattering (SR-DLS), the core technology behind the NanoFlowSizer, offers a direct solution to this challenge. Based on low-coherence interferometry, SR-DLS enables particle size measurements inside most sample containers, as long as light can pass through, making it suitable for virtually all plastic and glass packaging.
In this article, several examples are shown of how the NanoFlowSizer can be used to measure nanoparticles through a closed pharmaceutical packaging.
The NanoFlowSizer: Real-time analysis in closed containers
Pharmaceutical packaging plays a crucial role in maintaining the stability, safety, and compliance of pharmaceutical products. This packaging may be used for intermediate stages—where products still undergo further processing—or as final packaging for ready-to-use formulations.
Opening such packaging, however, can lead to changes in the physical or chemical properties of the product. These alterations may compromise product quality and affect downstream manufacturing steps or delay timely product release.
Traditional analysis methods often require time-consuming sample preparation, analysis, and data evaluation—factors that contribute to longer development timelines and increased production costs.
The NanoFlowSizer offers a powerful solution by enabling non-invasive, real-time measurement of nanoparticles in closed containers, without the need for sample preparation or dilution. It can operate dynamically or in a static mode, and is compatible with complex liquid formulations. These unique capabilities streamline process development, support quality assurance, and contribute to a more robust and efficient manufacturing process.
Measurement in a pre-filled syringe
Propofol emulsion was measured once by transferring the sample to a glass vial (in a destructive manner) and compared to the direct, non-destructive measurement directly in the prefilled syringe.
No significant difference was obtained between the two measurements.
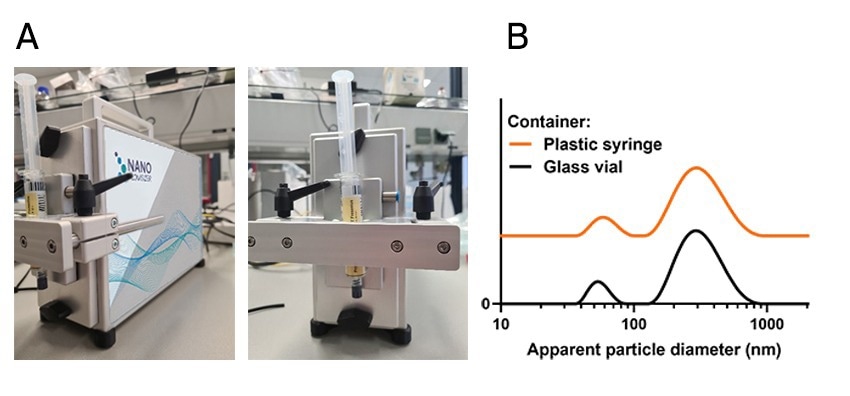
Figure 1. NanoFlowSizer measurement through a prefilled syringe. A) image of the NanoFlowSizer with the clamping module and syringe containing the propofol emulsion; B) Particle size result obtained by measurement through the plastic syringe, non-destructively (orange) and by conventional measurement in the glass vial (black). Image Credit: InProcess-LSP
Measurement through IV bag
The standard suspension of silica nanoparticles was measured directly through the IV bag (non-destructive manner) and compared to the sample measurement in a glass vial (standard, destructive DLS measurement).
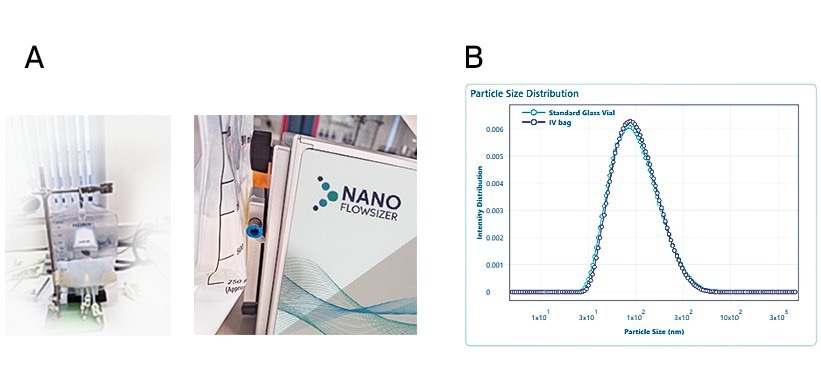
Figure 2. NanoFlowSizer measurement through an IV bag. A) image of the NanoFlowSizer with the clamping module and IV bag containing the 100 nm Silica nanoparticle standard solution suspended in water, B) Particle size result obtained by measurement through the IV bag, non-destructively (black) and by conventional measurement in the glass vial (blue). Image Credit: InProcess-LSP
Measurement through PET and PC 20L vessels
The standard suspension of silica nanoparticles was measured through two different plastic vessels (non-destructive manner) and compared to the sample measurement in a glass vial (standard, destructive DLS measurement). The overlapping of the Intensity-based PSD shows no difference among the different vessels used for the measurement. The Cum. Z-Average (nm) shows the same result (not shown).
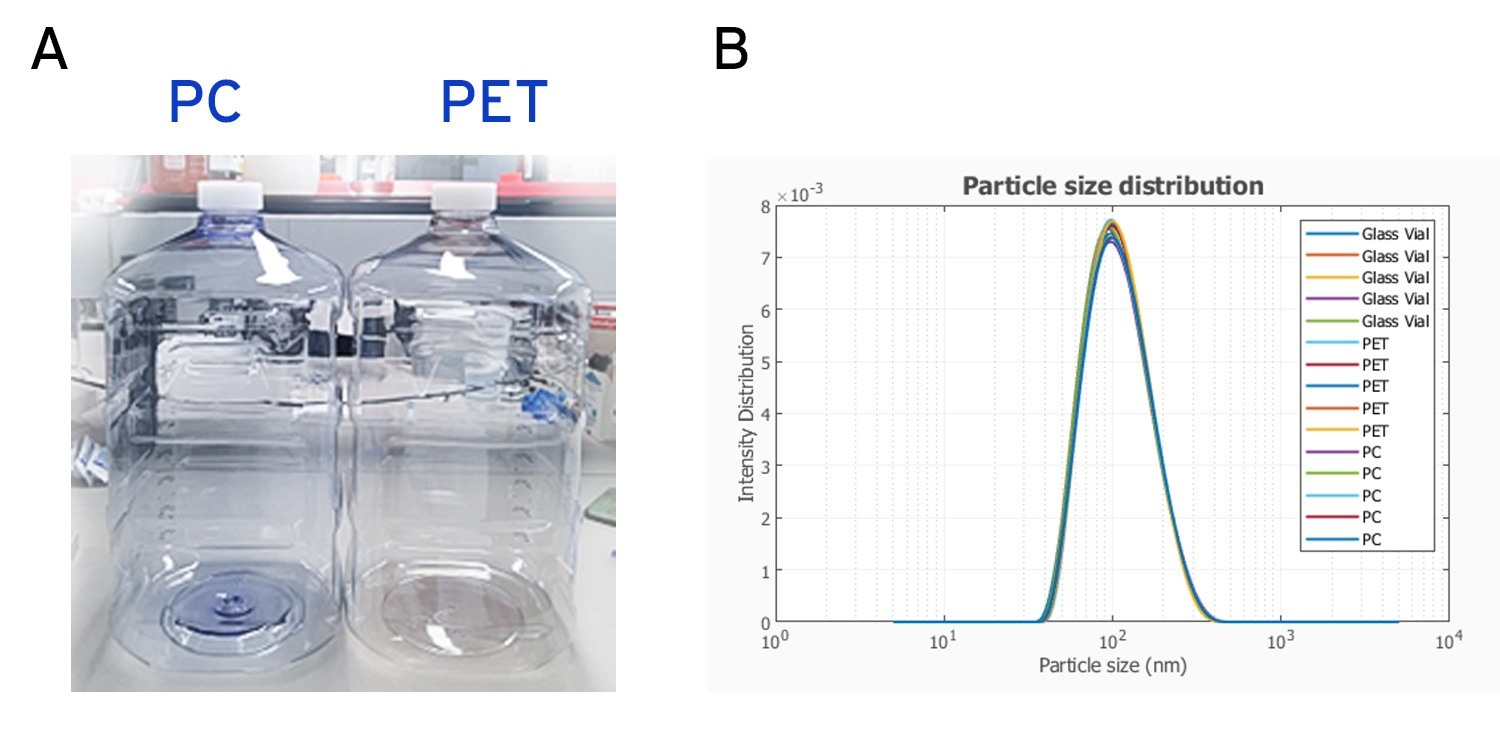
Figure 3. NanoFlowSizer through different types of bottles. A) Images of the Polycarbonate mixed4 Sure (PC) and PolyEthyleneTereftalate Mixed4Sure (PET) bottles; 100 nm Silica nanoparticle standard suspended in water was filled in the bottles and particle size measured by NanoFlowSizer B) Particle size result obtained by measurement through Polycarbonate mixed4 Sure bottle (PC), PolyEthyleneTereftalate Mixed4Sure bottle (PET) and in the glass vial (Glass Vial). Image Credit: InProcess-LSP
The NanoFlowSizer (SR-DLS)
The NanoFlowSizer is an analytical instrument to measure submicron particle size. The measurements are based on SR-DLS (Figure 4A).
SR-DLS (as well as standard DLS) is based on measuring the diffusion rate of particles suspended in a liquid by illuminating them and analyzing the fluctuations in scattered light signals at a given collection angle (Figure 4B). Suspended particles diffuse via Brownian motion, constantly changing their relative positions in the scattering volume. As a result, the scattered signal fluctuates at a rate that is proportional to the particle's diffusion rate.
By computing decorrelation functions of the scattered signal, their diffusion constant (or its distribution) can be measured, which can be translated into the hydrodynamic size and size dispersity of the diffusing particles via the Stokes-Einstein relation.
SR-DLS differs from conventional DLS in a number of ways: The collection angle is 180⁰ (full backscattering), which means that the same light path used to illuminate the sample is used to collect the scattered signal. In practice, full backscattering makes the measurements geometry independent, as measurements can be performed even through curved interfaces.
Secondly, SR-DLS implements Low Coherence Interferometry (LCI) methods. In LCI, broadband light illuminates the samples and is then mixed with the reference beam in an interferometer. From the measured spectrum of this mixed signal (over all wavelengths of the light source), the scattered signals from different depths in the sample can be resolved simultaneously.
Typically, 1000 consecutive depths with a resolution of a few microns and a total depth in the sample of a few millimeters are thus analyzed. At each depth, the signal is rapidly acquired, and a decorrelation function can be computed, from which particle size can be calculated (Figure 4C).
The ability of having a large number of correlation functions resolved in depth using a full backscattering optical set-up enables measurements in flowing pipes (through dedicated flow cells) and measurements at high turbidity in curved and complex product container geometries.
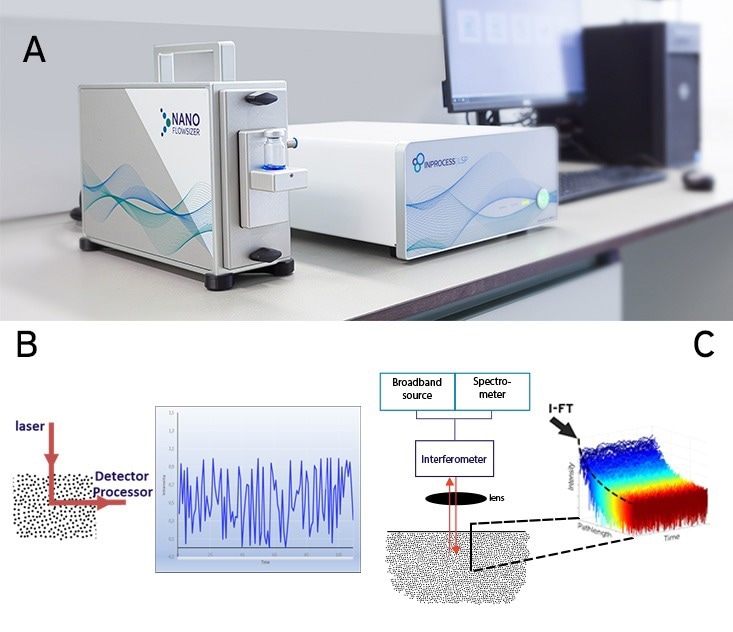
Figure 4. Spatially Resolved Dynamic Light Scattering (SR-DLS) A) The NanoFlowSizer set-up consists of a probe unit (left, equipped with a vial module), a base unit (middle) and a dedicated PC. B) In standard DLS measurements, the scattering signal of the illuminated volume is analyzed as a single large voxel, thus yielding a single time-dependent intensity signal that is then used to extract the corresponding particle size distribution. C) SR-DLS employs a different set-up to acquire spatially resolved data. Detail of the detection scheme: interferograms from the spectrometer are transformed to give the scattered light from each depth in the sample. High-speed acquisition thus yields fluctuations in this scattered light at different depths (blue: close to the wall, red: deeper in the suspension). Image Credit: InProcess-LSP
The NanoFlowSizer: Particle size tool for any closed container
Dedicated vial and clamp modules: Comfort and robustness
Pharmaceutical packaging comes in many forms and does not always fit the available modules for flasks. However, the modules are used with the objective of bringing the sample precisely to the measuring window. Consequently, if a sample can be placed at the correct position, it can be measured. We will give some examples of this concept using a basic module that can be placed in front of containers.
The NanoFlowSizer is a versatile, modular system to which the front module can be exchanged to accommodate the sample of interest. The main task of the modules is to ensure that samples sit at the right position to be measured.
Different commercially available modules are available and can be customized upon request to hold any other type of small flask (Figure 5). Particle size measurement can be done in different commercially available prefilled containers without disturbing the liquid within the container.
This unique feature of the NanoFlowSizer enables the rapid, at-line particle size characterization of finished pharmaceutical products without the necessity to open the sample container.

Figure 5. The NanoFlowSizer (NFS) and measurements inside typical laboratory containers. Adjustable clamp modules can be used for different holders used in the laboratory or manufacturing facilities. Both static and inflow configurations are possible. Image Credit: InProcess-LSP
Conclusion
The NanoFlowSizer can be used to characterize particle size inside closed containers of varying materials and geometry. This capability is made possible by the inherent properties of SR-DLS technology.
For most lab-scale containers, dedicated modules are available to accurately position the samples at the measurement window. With these configurations, common laboratory vials, glassware, and various packaged formulations can be analyzed effectively. For example, a prefilled syringe containing propofol was measured directly, producing its characteristic particle size distribution (PSD) fingerprint, even at very high turbidity.
For larger or irregularly shaped containers, flexible, non-specific modules can also be used with ease. For instance, particle size distribution (PSD) was successfully measured inside large plastic containers made of different materials (PET and PC), as well as in IV bags.
In summary, the NanoFlowSizer can measure the particle size distribution (PSD) and polydispersity index (PDI) of suspensions in nearly any transparent container, regardless of its geometry, using specialized modules. These modules can be custom-designed upon request—contact us to discuss your specific application and particle sizing requirements.
References
- Besseling, R., et al. (2019). New unique PAT method and instrument for real-time inline size characterization of concentrated, flowing nanosuspensions. European Journal of Pharmaceutical Sciences: Official Journal of the European Federation for Pharmaceutical Sciences, (online) 133, pp.205–213. https://doi.org/10.1016/j.ejps.2019.03.024.
- Costa, A.P., et al. (2015). Liposome Formation Using a Coaxial Turbulent Jet in Co-Flow. Pharmaceutical Research, 33(2), pp.404–416. https://doi.org/10.1007/s11095-015-1798-8.
- Einstein, A. (1905). On the motion of particles suspended in liquids at rest, as required by the molecular-kinetic theory of heat. Annalen der Physik , 322(8), pp. 549–560. https://doi.org/10.1002/andp.19053220806.
- Goodarzi, F. and Zendehboudi, S. (2018). A Comprehensive Review on Emulsions and Emulsion Stability in Chemical and Energy Industries. The Canadian Journal of Chemical Engineering, 97(1), pp.281–309. https://doi.org/10.1002/cjce.23336.
- Gupta, A., et al. (2016). Controlling and predicting droplet size of nanoemulsions: scaling relations with experimental validation. Soft Matter, (online) 12(5), pp.1452–1458. https://doi.org/10.1039/C5SM02051D.
- Håkansson, A. (2019). Emulsion Formation by Homogenization: Current Understanding and Future Perspectives. Annual Review of Food Science and Technology, 10(1), pp.239–258. https://doi.org/10.1146/annurev-food-032818-121501.
- Kalkman, J., Sprik, R. and van Leeuwen, T.G. (2010). Path-Length-Resolved Diffusive Particle Dynamics in Spectral-Domain Optical Coherence Tomography. Physical Review Letters, 105(19). https://doi.org/10.1103/physrevlett.105.198302.
- Nick Koumakis., et al. (2023). Instrument dependence of DLS particle size data: implications for data interpretation and standards measurements. ResearchGate . https://doi.org/10.13140/RG.2.2.14759.32168.
- Schuurmans, C.C.L., et al. (2022). Inline Particle Sizing in Flow for Demanding Nanosuspension Processes. ResearchGate. Available at: https://www.researchgate.net/profile/Rut-Besseling/publication/359691923_Inline_particle_sizing_in_flow_for_demanding_nanosuspension_processes/links/65450d2eb86a1d521bb44829/Inline-particle-sizing-in-flow-for-demanding-nanosuspension-processes.pdf (Accessed: 06 June 2025).
- Wikipedia Contributors (2023). Proportional–integral–derivative controller. (online) Wikipedia. Available at: https://en.wikipedia.org/wiki/Proportional%E2%80%93integral%E2%80%93derivative_controller.
About InProcess-LSP
InProcess-LSP, headquartered in Oss at Pivot Park, is a rapidly growing, innovative company founded in 2014. Backed by a team of in-house experts—comprising physicists, chemists, and software engineers—InProcess-LSP is at the forefront of nanotechnology solutions. The company’s leading product, the NanoFlowSizer, is a cutting-edge instrument designed to deliver inline, real-time measurements of nanoparticles in solution, making it indispensable across various industries.
Utilizing Spatially Resolved Dynamic Light Scattering (SR-DLS) technology, the NanoFlowSizer enables accurate characterization of nanoparticles in flowing liquids, providing critical data such as hydrodynamic diameter, polydispersity index (PDI), and D90 within seconds.
This state-of-the-art instrument empowers both scientists and industries by offering a robust solution for analyzing nanoparticle properties, paving the way for breakthroughs in product development, improved formulations, and pioneering applications.
Innovators in Process Analytical Technology and nanoparticle characterization.
With their strong background in process analytics as well as many years of academic and industrial experience InProcess offer a highly skilled and experienced team of scientists and process specialists addressing the needs of your PAT and nanotechnology challenges.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.