Sponsored Content by InProcess-LSPReviewed by Olivia FrostNov 13 2025
Polyethylenimine (PEI)-assisted transfection is a mainstay in upstream processing (USP) for viral vector manufacturing. But recent developments are shifting it from a costly, largely trial-and-error method into a more predictable and efficient process.
Improvements in PEI particle stabilization, new tools like Spatially Resolved–Dynamic Light Scattering (SR-DLS), and advances in automation are all helping bring better control over the particles that drive transient transfection. These changes are boosting transfection efficiency and consistency, while also cutting down on material use, hands-on time, and failed batches.
This report will highlight key developments in USP for transient transfection, specifically in the context of PEI-based methods.
While PEI-assisted transfection continues to be a foundational strategy for viral vector production, recent research and process innovations are shifting it from a largely empirical approach to one that is data-informed and operationally streamlined.1,2
The aim of this report is to inform you of the new developments in PEI-based transfection, focused on new insights from literature, new stabilizing additives for PEI-pDNA complex particles, and new innovations in automation and monitoring of transient transfection using PAT (Process Analytical Technology).
The importance of pDNA-PEI complex particle size in transient transfection
All PEI-enabled transfection of viral vector producer cells begins with the initial uptake of plasmid DNA (pDNA) facilitated by PEI.1,2 PEI binds to the plasmids to form nanoparticles that can be internalized by the cells.³ The formation and uptake of these PEI–pDNA complexes, or polyplexes, follow a sequence of well-characterized physicochemical and biological steps.3
The process starts with the rapid electrostatic interaction between the positively charged amines on PEI and the negatively charged phosphate backbone of the plasmid DNA, leading to the condensation of the DNA into nanoscale complexes (Step 1 in Figure 1). The resulting particle’s size, charge, and uniformity depend on several factors - including the nitrogen-to-phosphate (N/P) ratio, buffer conditions, mixing behavior, and especially the time after complexation.
Once formed, these positively charged polyplexes interact with cell-surface proteoglycans and are taken up by the cell through various endocytic pathways, primarily clathrin- and caveolin-mediated endocytosis, as well as macropinocytosis (Step 2 in Figure 1). Inside the endosome, PEI is thought to trigger the “proton sponge” effect due to its high buffering capacity. This leads to osmotic swelling and eventual rupture of the endosome, releasing the pDNA into the cytoplasm (Step 3 in Figure 1).
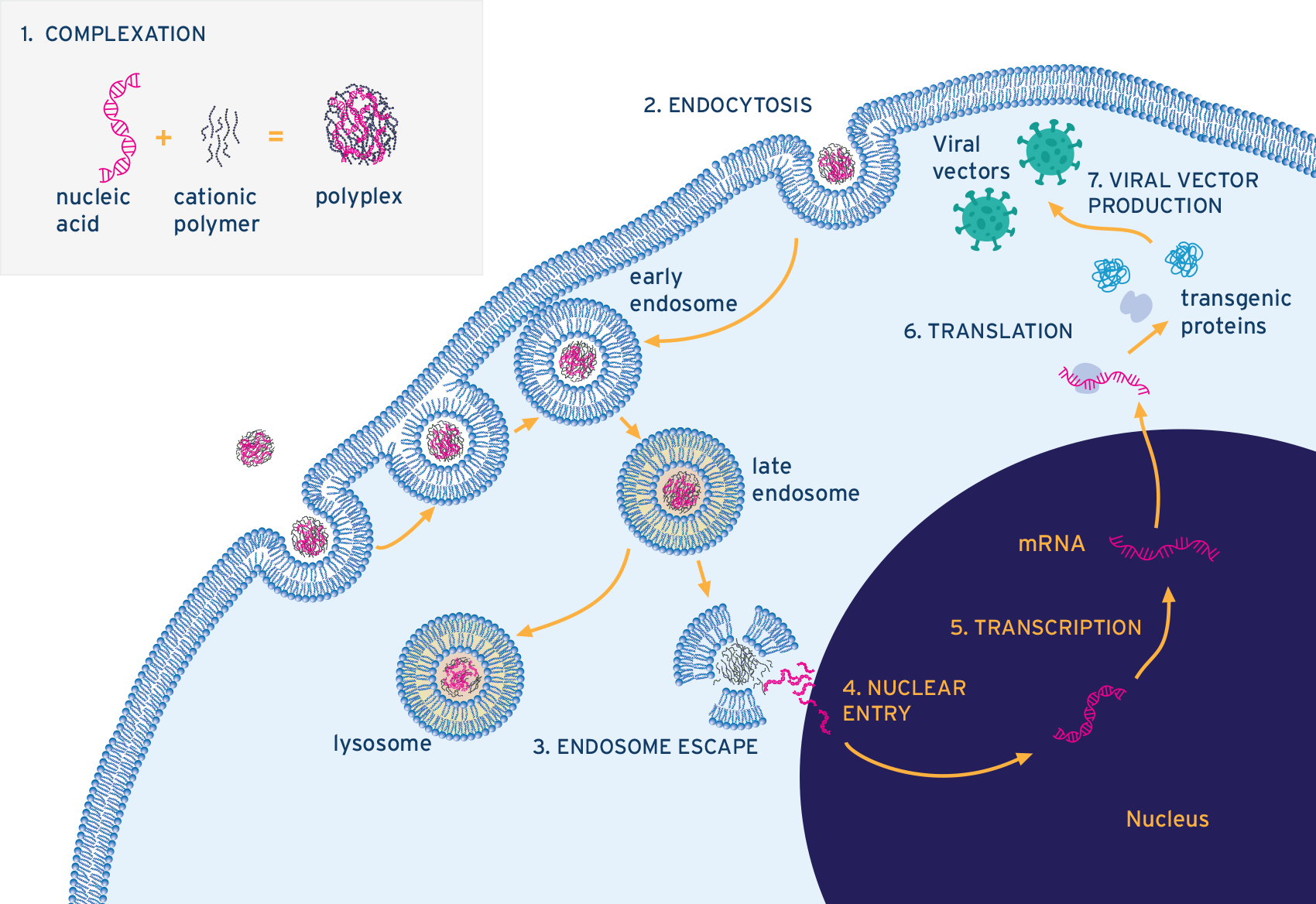
Figure 1. Diagram showing the steps involved in the formation and internalisation of pDNA-PEI complexes into viral vector-producing cells. Image Credit: InProcess-LSP
Following endosomal escape, partial dissociation of the PEI polymer allows the plasmid DNA to be released into the cytoplasm. From there, trafficking toward the nucleus occurs either via microtubule-mediated transport or during mitosis, when the nuclear envelope temporarily breaks down (Step 4 in Figure 1). Once inside the nucleus, the plasmid can be transcribed, initiating expression of the therapeutic transgene and completing the transfection process.
With the transgene now active, the cell is equipped to produce viral vectors through subsequent rounds of transcription, translation, and viral assembly (Steps 4, 5, and 6 in Figure 1).
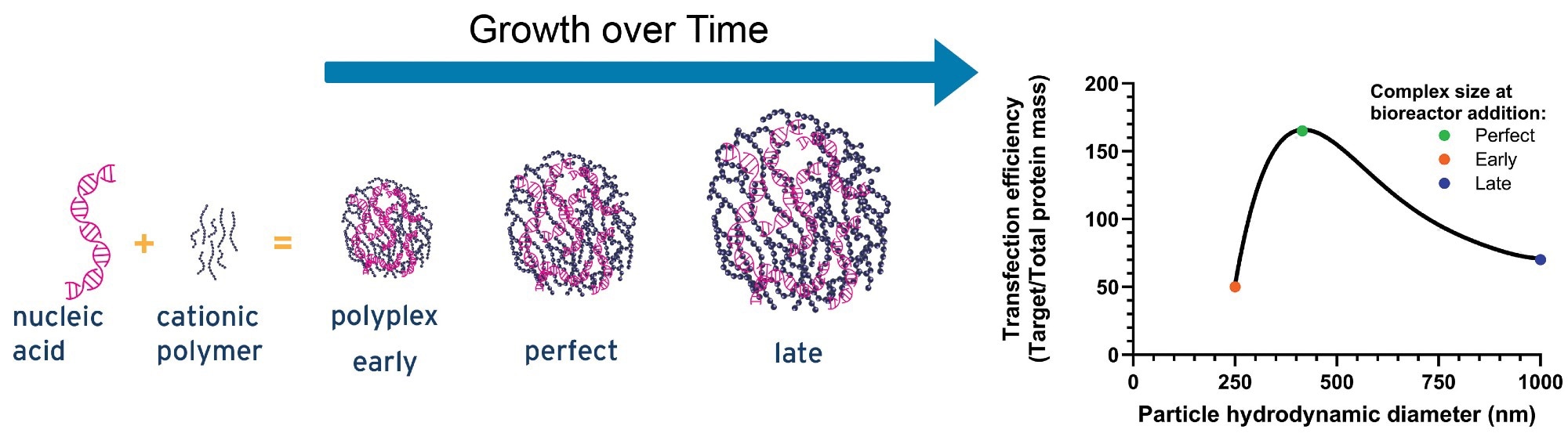
Figure 2. An illustrative graph showing the influence of pDNA-PEI complex size on transfection efficiency4. Image Credit: InProcess-LSP
Overall, the efficiency of each step, from nanoscale condensation to final vector production, depends heavily on the initial size and charge of the PEI–pDNA complexes. These early formulation parameters directly influence key transfection outcomes, including cell viability, total vector titer, full-to-empty particle ratios, and, ultimately, consistent viral vector yield.
Optimizing the size of these complexes is especially important. Both overly small and overly large particles can reduce transfection performance.4 Larger particles are more prone to poor cellular uptake, sedimentation, or aggregation, while smaller ones may struggle with effective endosomal escape or risk releasing DNA prematurely. Research suggests there’s a narrow size window that supports optimal gene expression; deviating from it in either direction can reduce transfection efficiency by as much as threefold (see illustrative graph in Figure 2).
As a result, controlling the hydrodynamic diameter during the formation of PEI–pDNA complexes has become a critical factor for improving yield, reproducibility, and cost-efficiency in gene therapy vector production.
How do I control complex size? – Trends in PEI-driven transient transfection
PEI remains one of the most widely used and effective non-viral transfection reagents, valued for its high cationic charge density and strong ability to condense nucleic acids. However, there’s been a notable shift in recent years from relying on PEI as a standalone driver of transient transfection to developing engineered, stabilized, and application-specific systems that address its longstanding drawbacks: cytotoxicity, batch-to-batch variability, and instability of PEI–pDNA complexes.
Today’s next-generation PEI-based transfection platforms combine chemical optimization, formulation advances, and process engineering to deliver scalable, consistent, and clinically relevant solutions. Among the most significant advancements is the development of stabilizing additives that enhance complex formation and extend the shelf-life of PEI–nucleic acid mixtures.
Traditionally, PEI-based complexes required precise, fast mixing and immediate use, as they were prone to aggregation or loss of activity within minutes. Emerging stabilizers (such as those developed by Mirus Bio or the Mao group at Johns Hopkins in collaboration with Sartorius Polyplus)5,6,7,8 significantly extend this usable window. They enable the formation of complexes over longer timeframes, help lock in optimal particle size, and allow for formulation at larger reaction volumes without sacrificing transfection efficiency or viral yield.
These stabilizers typically work by tuning the ionic environment to prevent premature aggregation, resulting in a more uniform and stable particle population. This has important implications for large-scale production of viral vectors and plasmid DNA, where consistency and scalability are both critical and difficult to achieve.
SR-DLS: The most efficient solution for real-time particle size monitoring
Spatially Resolved Dynamic Light Scattering (SR-DLS) is a technology introduced by InProcess-LSP in 2019. The commercially available NanoFlowSizer instrument, which leverages SR-DLS, serves as a process analytical technology (PAT) solution for real-time, non-destructive particle size analysis - primarily used for flowing nanosuspensions 9,10.
Looking ahead to 2026, a new dedicated offline system based on SR-DLS and enhanced with imaging capabilities is set to launch, designed specifically to meet the evolving needs of early- and late-stage transfection process development. This upcoming system will be the first of its kind to measure particle size in viral vector upstream processing (USP) continuously during pDNA–PEI complex formation without disrupting sterility or requiring sampling.
Its non-invasive, geometry-independent design allows continuous, real-time monitoring during mixing. This makes it possible to accurately determine the moment when complexes reach their optimal hydrodynamic diameter for transfection (see representative complex growth curves in Figure 3, left).
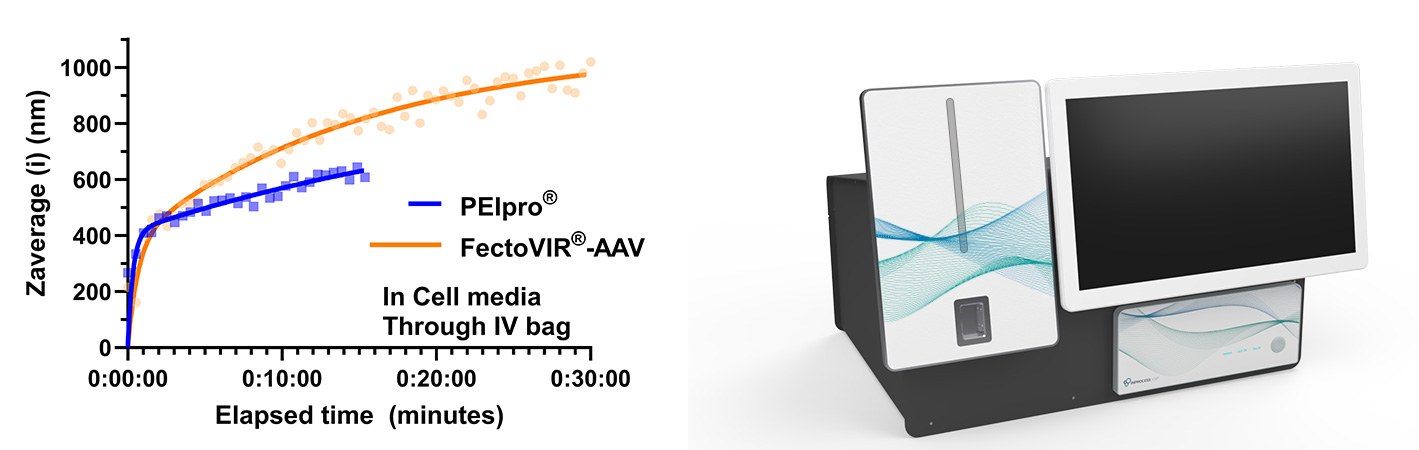
Figure 3. Left: SR-DLS data of representative pDNA-PEI complex growth based on a standard protocol of 3-plasmids mixed with existing PEI-based products according to supplier instructions. Right: dedicated offline SR-DLS system for transfection studies (commercially available in 2026). Image Credit: InProcess-LSP
Unlike the existing NanoFlowSizer PAT instrument, which is designed for in-line, flow-based measurements, the upcoming offline SR-DLS system will be a more compact solution optimized for monitoring dynamic processes in static samples. Its flexibility is one of its key strengths, as it can accommodate a wide range of volumes (from less than a milliliter to 3 liters) and is compatible with various container types, including vials, Falcon tubes, flasks of different sizes, and single-use bags up to 3 liters.
This versatility makes it particularly well-suited for applications ranging from early-stage research to mid-scale clinical batch development.
Benefits of complex size monitoring in USP transfection
1. R&D / Process Development
To justify investment in new technology, a clear business case is essential. In this context, the key question is: “What value does non-invasive, real-time complex size monitoring add to transfection in upstream processing?”
In R&D and process development, the benefits of continuous SR-DLS monitoring can be grouped into a few main categories:
- Deeper process understanding:
- Helps to accelerate development, scale-up, and validation
- Enables a direct link between complex size and batch performance (e.g., yield, titer).
- Provides a rich, continuous size-growth curve rather than snapshot data from traditional offline methods
- Delivers representative particle size data by eliminating sampling artifacts and time delays
- Greater efficiency:
- Reduces total development time, number of experiments, and hands-on labor
- Replaces destructive sampling and DLS of dedicated “sacrifice” batches used to generate size-growth profiles
- Continuous kinetic data typically lowers the number of required transfections
- Convenience:
- No need for skilled lab personnel to record SR-DLS size curve (no handling of sample required)
- Direct savings:
- Reduces materials, waste, batch numbers, and labor requirements
- Avoids the need to run separate non-sterile batches for offline complex size analysis
- Enables titer/yield increases of 15–30% by optimizing transfection timing and composition
- Cuts the use of sampling disposables and time spent on manual measurements
Realistically quantifying savings depends on several input parameters, but insights from leading gene therapy companies suggest annual program savings of €200,000–€500,000. The largest financial impact comes from avoiding repeat batches and increasing yield at meaningful scales (e.g., clinical studies). Time and compliance savings, especially during validation, engineering batches, and tech transfers, also contribute.
To put this into perspective: skipping a single 50 L development batch can save approximately €100,000–€200,000.
2. Commercial production
In commercial PEI-based viral vector production, real-time particle size monitoring supports process automation and control. The SR-DLS instrument can signal the precise moment to add stabilizers to prevent aggregation or identify the optimal point to transfer complexes into the bioreactor.
As a Process Analytical Technology (PAT) tool, SR-DLS directly connects nanoscale complex formation to process performance, improving reproducibility, minimizing material loss, and enhancing overall efficiency. While the offline SR-DLS system isn't intended for high-volume manufacturing (above ~500 L), it can still deliver substantial benefits, especially when stabilizers are part of the process.
At large scales, the time required to transfer complexes to cells can exceed 20 minutes, making precise timing more challenging. Here, stabilizers extend the working window, while real-time SR-DLS monitoring ensures accurate stabilizer addition and confirms size stabilization. These steps can be automated, reducing variability and improving yield.
Even modest improvements in yield - 15 % to 30 % - can translate into major financial gains. For an average commercial production site, this could amount to €7 million in annual savings.
References
- Ehrhardt, C., et al. (2006). Polyethylenimine, a cost-effective transfection reagent. Signal Transduction, 6(3), pp.179–184. DOI: 10.1002/sita.200500073. https://onlinelibrary.wiley.com/doi/abs/10.1002/sita.200500073
- Lorsch, J. (2013). Laboratory methods in enzymology : DNA. Amsterdam ; Boston: Elsevier. https://www.sciencedirect.com/science/article/abs/pii/B9780124186873000185
- Midoux, P., et al. (2008). Polymer-Based Gene Delivery: A Current Review on the Uptake and Intracellular Trafficking of Polyplexes. Current Gene Therapy, 8(5), pp.335–352. DOI: 10.2174/156652308786071014. https://www.benthamdirect.com/content/journals/cgt/10.2174/156652308786071014.
- Hu, Y., et al. (2024). Liter-scale manufacturing of shelf-stable plasmid DNA/PEI transfection particles for viral vector production. Molecular therapy. Methods & clinical development, 32(1), pp.101194–101194. DOI: 10.1016/j.omtm.2024.101194. https://www.cell.com/molecular-therapy-family/methods/fulltext/S2329-0501(24)00010-X
- Eş, I. (2025). Microfluidic synthesis of PEI/plasmid DNA polyplexes for downstream recombinant AAV vector synthesis: Physico-chemical and in vitro transfection study. Chemical Engineering Science, 317, p.122075. DOI: 10.1016/j.ces.2025.122075. https://www.sciencedirect.com/science/article/abs/pii/S000925092500898X
- Lin, J., et al. (2025). Buffer Valency Engineering Enables High-concentration and Shelf-stable DNA Transfection Particles for Viral Vector Production. bioRxiv. DOI: 10.1101/2025.07.02.662322. https://www.biorxiv.org/content/10.1101/2025.07.02.662322v1.abstract
- US12161766B2 (2023). Methods of preparing polyelectrolyte complex nanoparticles - Google Patents. [online] Available at: https://patents.google.com/patent/US12161766B2/en [Accessed 12 Nov. 2025].
- US20240115513A1 (2022). Methods for preparation of plasmid dna/lipid particles with defined size for in vitro and in vivo transfection - Google Patents. [online] Available at: https://patents.google.com/patent/US20240115513A1/en [Accessed 12 Nov. 2025]. https://patents.google.com/patent/US20240115513A1/en
- US11555772B2 (2017). Method and apparatus for in-process particle size determination of nanosuspensions under flow. Google Patents. [online] Available at: https://patents.google.com/patent/US11555772B2/en
- Besseling, R., et al. (2019). New unique PAT method and instrument for real-time inline size characterization of concentrated, flowing nanosuspensions. European Journal of Pharmaceutical Sciences: Official Journal of the European Federation for Pharmaceutical Sciences, 133, pp.205–213. DOI: 10.1016/j.ejps.2019.03.024. https://www.sciencedirect.com/science/article/abs/pii/S0928098719301332
About InProcess-LSP
InProcess-LSP, headquartered in Oss at Pivot Park, is a rapidly growing, innovative company founded in 2014. Backed by a team of in-house experts - comprising physicists, chemists, and software engineers - InProcess-LSP is at the forefront of nanotechnology solutions. The company’s leading product, the NanoFlowSizer, is a cutting-edge instrument designed to deliver inline, real-time measurements of nanoparticles in solution, making it indispensable across various industries.
Utilizing Spatially Resolved Dynamic Light Scattering (SR-DLS) technology, the NanoFlowSizer enables accurate characterization of nanoparticles in flowing liquids, providing critical data such as hydrodynamic diameter, polydispersity index (PDI), and D90 within seconds.
This state-of-the-art instrument empowers both scientists and industries by offering a robust solution for analyzing nanoparticle properties, paving the way for breakthroughs in product development, improved formulations, and pioneering applications.
Innovators in process analytical technology and nanoparticle characterization.
With their strong background in process analytics as well as many years of academic and industrial experience InProcess offer a highly skilled and experienced team of scientists and process specialists addressing the needs of your PAT and nanotechnology challenges.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.