Sponsored Content by LittelfuseReviewed by Olivia FrostSep 19 2025
The rapid progress of IoT and compact electronics is transforming the medical device scene, with one of the most significant advancements being the wearable medicine injector.

Image Credit: raker/Shutterstock.com
For patients who need regular drug delivery and continuous monitoring, these devices significantly improve their quality of life.
Instead of being connected to huge, line-powered devices, patients can move freely while their therapy is ongoing in the background.
Wireless connectivity provides medical experts with real-time access to treatment data, allowing for dosage modifications or flow rate changes without the patient attending a clinic.
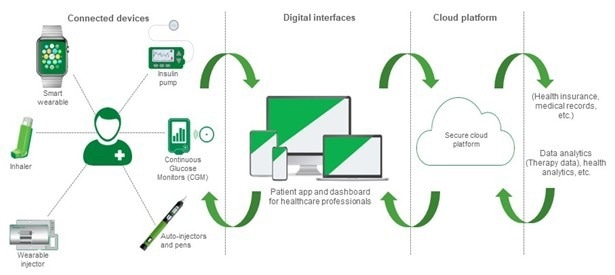
Figure 1. Connected drug delivery systems are revolutionizing the way patients receive medications. Image Credit: Littelfuse
Behind this patient convenience lies a complex engineering problem. Wearable injectors must function consistently around the clock, seven days a week. They must do so with little energy usage to maximize battery life while remaining lightweight and comfortable to wear.
Designers must combine compact, efficient, and dependable components into a small form factor while adhering to tight medical safety rules.
This article examines techniques for achieving such objectives, with a particular emphasis on circuit protection against electrical hazards, including overcurrent and electrostatic discharge (ESD).
It also explores modern sensing and detection technologies that allow designers to cut power consumption without sacrificing precision. Together, these measures help to ensure that wearable injectors can provide safe and effective therapy in real-world settings.
Understanding the wearable injector architecture
Before looking into component selection, it is helpful to visualize the entire system.
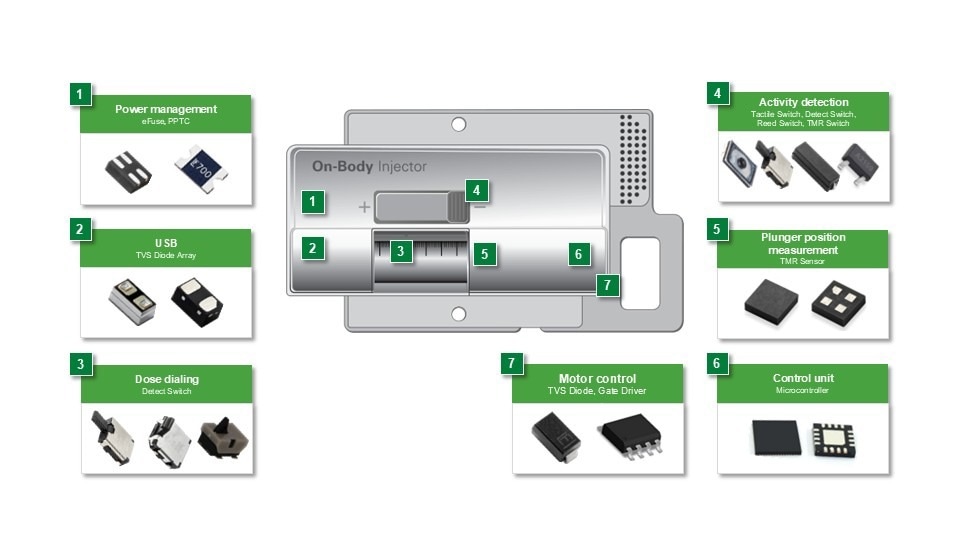
Figure 2. Wearable medicine injector example and recommended protection and control components. Image Credit: Littelfuse
A wearable injector consists of several interdependent circuit blocks, each with its own protection and control scheme.
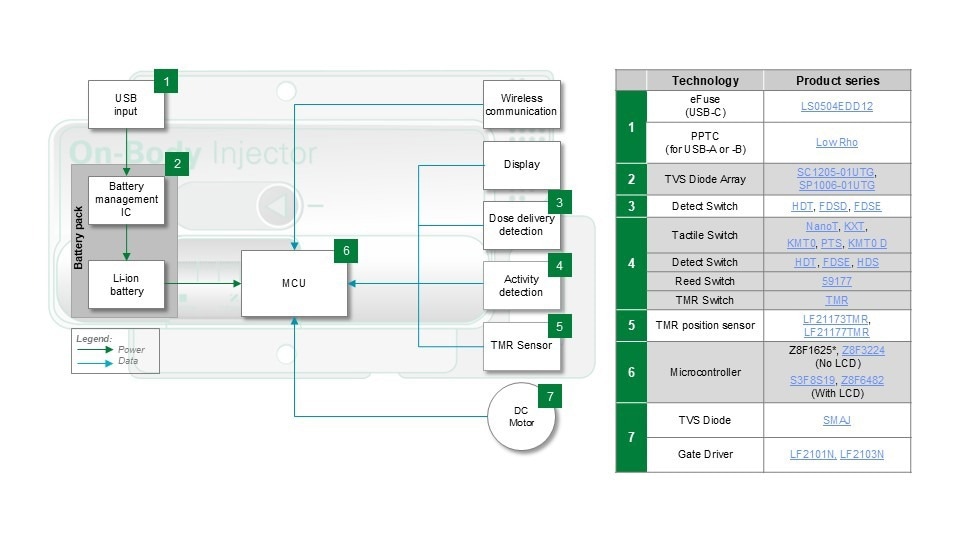
Figure 3. Wearable medicine injector block diagram. Image Credit: Littelfuse
Figure 3's block diagram shows that the injector's principal energy source is a rechargeable lithium-ion (Li-ion) battery. Charging occurs via a USB port. The injector's power requirements determine which USB interface - A, B, or C - is used.
USB-A and USB-B can deliver up to 20 W, which is suitable for many devices; however, USB-C can provide up to 100 W for more demanding applications. The microcontroller (MCU) is at the heart of the system. It controls the injector's motor, receives feedback from position and activity sensors, and handles wireless connection.
This wireless interface is critical for modern linked care since it enables doctors to track treatment progress, check patient adherence, and remotely alter settings. With so much functionality packed into such a small space, safety and efficiency become design priorities.
Building in protection for reliability
Strong protection against electrical risks is essential for continuous, safe operation. A few carefully selected protection components can provide dependability while reducing board area and energy usage.
Protecting USB charging inputs
Polymer positive temperature coefficient (PPTC) resettable fuses offer reliable overcurrent protection for designs that charge via USB-A or USB-B. These fuses automatically reset once a fault is cleared, eliminating the need for human replacement.
The low resistance levels (typically less than 1 Ω) reduce voltage loss and wasted power. Their availability in small surface-mount packaging makes them an ideal choice for portable electronics.
Higher-power USB-C inputs necessitate more thorough approaches. Integrated eFuse ICs incorporate numerous levels of security into a single chip.
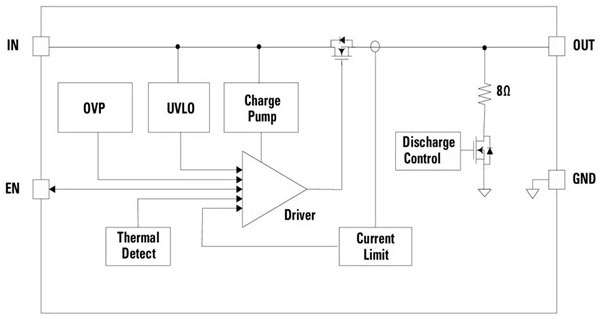
Figure 4. LS0504EDD12 5.5 V, 4 A Low-RON eFuse Load Switch. Image Credit: Courtesy of Littelfuse
An eFuse protects against overcurrent, overvoltage, and overtemperature circumstances. Additional characteristics include soft-start to reduce inrush current, undervoltage lockout to prohibit operation at low supply levels, and low on-resistance (26 mΩ) to preserve energy.
The device is packaged in a 1.2 x 1.6 mm DFN format, making it suitable for wearable applications without compromising performance.
Safeguarding the battery management circuit
Battery longevity is critical to the patient experience. A dedicated battery management IC monitors the status of charge (SoC) and health (SoH). To protect this delicate circuit, designers frequently use TVS diode arrays.
A bidirectional array with two anode-to-anode diodes can safely withstand ESD occurrences up to ±30 kV. With millisecond response times and clamping voltages of roughly 10 V, these devices protect the battery management IC from harm while maintaining performance.
Motor drive circuit protection
The motor driving circuit generates the mechanical action of the injector plunger. This circuit requires strong protection due to its susceptibility to surges and ESD occurrences. Surface-mount TVS diodes are ideal for this application, as they can absorb up to 400 W of surge power and survive ESD strikes up to 30 kV.
Their ultra-fast response time of less than 1 ps protects delicate semiconductor components. Depending on the circuit architecture, unidirectional and bidirectional choices provide versatility.
Protecting communication and display interfaces
ESD can interfere with displays and wireless communication modules. Low-capacitance TVS diodes are required to protect them, suppressing ESD while not distorting RF signals. This method ensures accurate data transmission and avoids errors that could jeopardize patient safety.
When combined, these compact, surface-mount devices provide comprehensive protection with little energy consumption. They protect the injector from real-world electrical threats while maintaining the low power consumption that wearable devices require.
Sensing and control for efficient operation
In addition to protection, efficient sensing and control are essential for reducing power consumption and maintaining proper operation. Designers have a variety of technologies to select from, each with unique trade-offs in terms of size, sensitivity, and energy usage.
Dose delivery detection
It is critical to accurately identify the dosage dial's location. Compact surface-mount detect switches can accomplish this with a small footprint.
These compact devices, measuring 3.5 × 2.8 × 3.35 mm, have a low actuation force of 35 g, contact resistance of 500 mΩ, and can sustain over 100,000 cycles. The ability to actuate from the top or side allows for greater design flexibility.
Activity detection options
Activity detection performs multiple roles, including identifying the presence of a drug vial, verifying injector-to-skin contact, commencing the injection, and monitoring the given doses. There are several sensing technologies available.
- Pushbutton switches: Surface-mounted, low-profile pushbutton switches (as compact as 2.5 × 1.65 × 0.55 mm) with IP67 moisture and dust resistance. With lives of up to 300,000 cycles and minimal contact resistance, they provide dependable performance in severe environments.
- Detect switches: Similar to those used for dose detection, these are compact and durable.
- Reed switches: Reed switches are hermetically sealed, ultra-miniature electronics controlled by magnetic fields. They require no standby power and leak only picoamps of current, making them extremely energy efficient.
- Tunneling magnetoresistance (TMR) switches: These switches are advanced devices that combine excellent sensitivity with low power consumption.
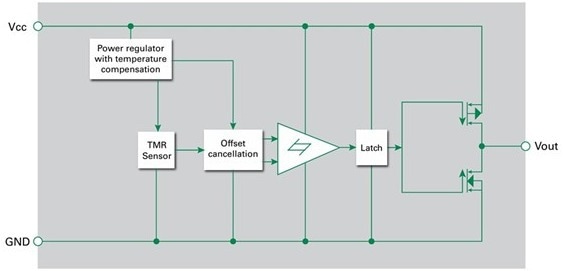
Figure 5. Integrated switch with tunneling magnetoresistance sensor and drive circuitry. Image Credit: Courtesy of Littelfuse
TMR switches show special potential for monitoring plunger position. They are positioned toward the bottom of the injector chamber and detect the approach of a compact magnet installed on the plunger.
As the motor moves the plunger, the switch output changes state, providing accurate position feedback. The MCU then utilizes this data to compute the volume of the administered medication.
TMR switches are extremely efficient, drawing only 200 nA when driven from 1.8 to 5 V and being sensitive to fields as low as 5 Gauss. Their CMOS output provides a direct MCU interface without the need for extra hardware, making them an excellent choice for monitoring dose in battery-powered devices.
Control electronics
At the system level, the MCU coordinates motor control, sensing, and wireless communication. Efficiency is paramount. MCUs may run at currents as low as 2 mA and drop to 0.7 µA in inactive modes.
They may also have 12-bit ADC channels, on-chip temperature monitoring, 16 kbytes of flash memory, and high-resolution timers for precise motor control. Gate driver ICs are commonly used to connect the MCU to the motor's power transistors.
These drivers provide accurate, efficient motor control, ensuring the injector maintains consistent torque and reliable performance. They have TTL and CMOS-compatible inputs, Schmitt-triggered circuitry to decrease noise, and propagation delays as short as 50 ns.
Navigating regulatory standards
Because wearable injectors are medical devices, regulatory compliance is unavoidable. Engineers must design with applicable standards in mind from the beginning.
Table 1. Applicable standards for wearable medicine injectors. Source: Littelfuse
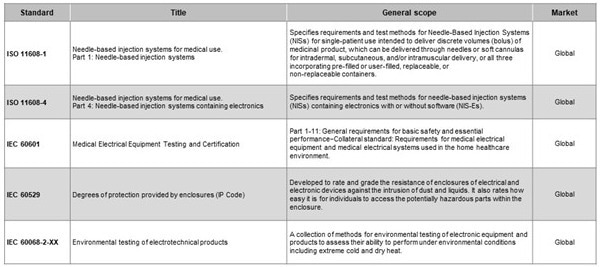
These standards govern issues ranging from electrical safety to biocompatibility. Integrating them into component selection and system architecture assures patient safety and speeds up the regulatory approval process. Early consideration can help avoid costly redesigns or delays caused by test failures.
Designing for reliability, efficiency, and patient safety
The engineers' aim is clear: wearable medicine injectors must provide continuous, safe operation in a compact, lightweight, and energy-efficient container. Protection against overcurrent, overvoltage, overtemperature, and ESD is critical for dependability.
At the same time, efficient sensor and control technologies allow for lower power consumption and longer battery life.
Partnering with skilled component makers can help to streamline the process. Suppliers frequently provide application engineering support, information on standards, and even pre-compliance testing. This knowledge helps to accelerate development, reduce risks, and avoid certification delays.
Engineers can create wearable injectors that meet the highest safety and performance standards by incorporating robust protection and low-power sensing into their designs - devices capable of running continuously 24/7, improving treatment outcomes, and providing patients with greater independence in their daily lives.
Reference literature
- Littelfuse. (2025). Littelfuse Catalogs, Circuit Protection Product Selection Guide. (online) Available at: https://electronicscatalogs.littelfuse.com/Circuit-Protection-Product-Selection-Guide/1/.
- Littelfuse. (2025). Littelfuse Catalogs, Sensing Products Selection Guide. (online) Available at: https://electronicscatalogs.littelfuse.com/Sensing-Products-Selection-Guide/30/.
- Littelfuse. (2016). Portable Medical Devices Protection Quick Reference Guide. (online) Available at: https://www.littelfuse.com/assetdocs/portable-medical-devices-protection-quick-reference-application-guide?assetguid=d1155aa0-e2af-406c-843b-ee953d256fb6
- C&K Components, Inc. (2022). Switch Solutions for Medical Applications, Market Flyer. https://www.ckswitches.com/media/3693/ck_medical-flyer.pdf.
Acknowledgments
This article was produced using materials originally written by Marco Doms at Littelfuse, Inc.
About Littelfuse
Littelfuse is a diversified industrial technology manufacturing company empowering a sustainable, connected, and safer world. Across more than 20 countries, and with approximately 16,000 global associates, we partner with customers to design and deliver innovative, reliable solutions. Serving over 100,000 end customers, our products are found in a variety of industrial, transportation, and electronics end markets - everywhere, every day. Headquartered in Chicago, Illinois, United States, Littelfuse was founded in 1927.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.