Sponsored Content by LittelfuseReviewed by Olivia FrostSep 10 2025
As healthcare shifts away from centralized models, there is an increasing need for portable, connected medical devices that work reliably beyond clinics. Drug delivery pens are central to this shift, helping patients self-administer medicines with accuracy and ease. However, designing them is complicated and presents significant challenges.

Image Credit: Littelfuse
There are three key factors to consider when designing drug delivery pens: accurate monitoring of drug administration, minimizing the device's physical size for patient comfort and portability, and extending battery life by keeping power consumption exceptionally low.
Growing demand for real-time patient data makes wireless connectivity of these devices essential, but it's difficult to integrate advanced electronics without compromising on size or reliability.
But how are smart component choices affecting the next generation of this med tech? The following strategies offer a solid foundation for drug delivery pen designs, from minimizing power and saving space to enabling seamless communication.
Designing an electronic drug delivery pen
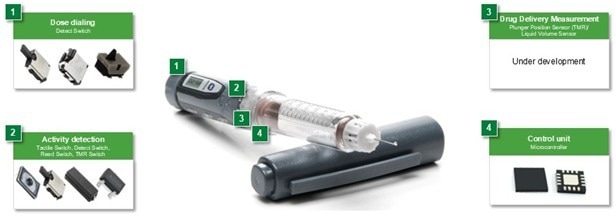
Figure 1. An electronic drug delivery pen and components that provide efficient control. Image Credit: courtesy of Littelfuse
A drug delivery pen's circuitry is powered by a battery. Depending on the ideal design, different battery types have advantages and disadvantages. For example, a lithium-ion coin cell is great for minimal space consumption.
A compact switch integrated into the device permits detection of the dosage dial position. Additional switches ensure the cap is removed to expose the needle, the needle is in contact with the user's skin, and the correct quantity of medication is delivered.
The microcontroller (MCU) receives inputs from the switches and drives the actuator, which injects a drug dose. There are several choices for an actuator. A spring actuator is advantageous in comparison to a small DC motor, as it reduces cost and complexity compared to a motor drive.
The MCU also transmits data to the display and interfaces with the Bluetooth circuit to transmit injection information and receive setting adjustments from remote medical personnel.
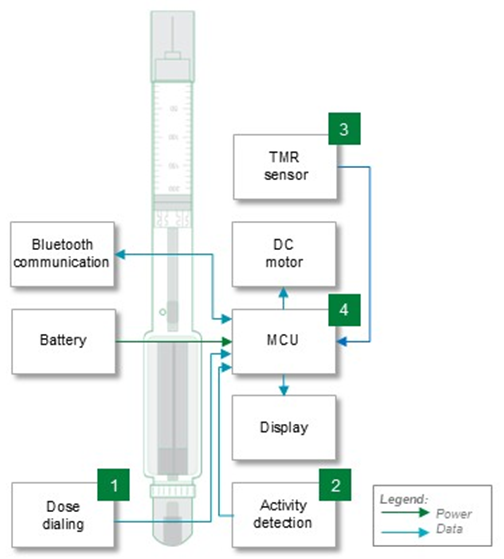
Figure 2. Electronic drug delivery pen block diagram. Image Credit: courtesy of Littelfuse
Source: Littelfuse
| |
Technology |
Product series |
| 1 |
Detect Switch |
HDT, FDSD, FDSE |
| 2 |
Tactile Switch |
NanoT, KXT, KMTQ, PTS, KMTO D |
| Detect Switch |
HDT, FOSE, HDS |
| Reed Switch |
59177 |
| MR Switch |
TMR |
| 3 |
Plunger Position Sensor (TMR) |
Under development; contact sales |
| Liquid Volume Measurement |
Under development; contact sales |
| 4 |
Microcontroller |
Z8F1625*, Z8F3224 (No LCD)
S3F8S19, Z8F6482 (With LCD) |
* Z8F1625 is undergoing development.
Efficient, compact control
Minimizing power consumption and space requires efficient sensing and control circuit blocks. The following options will help to achieve these properties in a design.
Dose dialing circuit
The Dose Dialing circuit requires a switch to indicate the position of the dose dial. Surface-mount detect switches as small as 3.5 mm wide x 2.8 mm deep x 3.35 mm high are available, and have a low activation force of 35 g. These switches have a low momentary contact resistance of 500 mΩ and a life exceeding 100,000 cycles. They can be activated either on top or on the side of the device.
Activity detection circuit
The Activity Detection circuit ensures safety and accuracy by confirming the drug vial's presence, verifying skin contact, activating injection, and monitoring the dose. Designers have several sensing options:
One option is a small, low-profile, top-actuated, surface-mount pushbutton switch, 2.5 mm wide x 1.65 mm deep x 0.55 mm high. Sealed switches are rated IP67 against ingress of moisture and dust. These pushbutton switches can have life cycles as high as 300,000 cycles, and contact resistance is low at 500 mΩ.
A second option is the type of detect switch suggested for the Dose Dialing circuit.
A third option is an ultra-miniature reed switch. Hermetically sealed and magnetically actuated, it draws no power and keeps leakage currents extremely low. The insulation resistance is 1012 Ω, which holds leakage current to pA levels.
Finally, tunneling magnetoresistance (TMR) switches offer high sensitivity and ultra-low power by combining a magnetic tunneling sensor with CMOS circuitry for efficient digital switching. Figure 3 shows a block diagram of a TMR switch.
TMR Switch
A temperature-compensated regulator circuit ensures voltage stability for the internal circuits. A Schmitt trigger provides switching hysteresis for noise rejection, and a CMOS push-pull circuit provides the output drive.
The TMR switch is positioned near the base of the injector's plunger chamber, with a small magnet attached to the plunger itself. As the actuator drives the plunger downward, closer to the TMR switch, the switch output voltage rises and its logic state changes. This signals the plunger's position, allowing the microcontroller to calculate the injected drug volume.
The TMR switch draws only 200 nA when powered by 1.8-5 V circuits. Its sensitivity can detect a magnetic field as low as five Gauss. The CMOS output enables direct connection to an MCU, which avoids the need for additional components. The TMR switch is available in very small SOT23-3 or LGA-4 packages. Its high sensitivity, compact size, and low battery drain make it a superior solution for drug dose monitoring.
Also, continuous glucose monitors (CGMs), when interacting with a drug delivery pen, can use TMR switches to support an optimum therapy protocol.
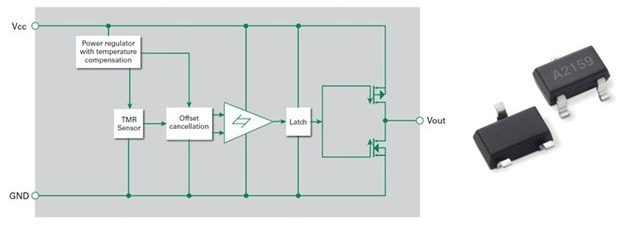
Figure 3. Integrated switch containing a tunneling magnetoresistance sensor and drive circuitry. Image Credit: courtesy of Littelfuse
TMR Sensor
Two technologies are under development for direct measurement of injected liquid volume.
One approach uses an advanced TMR sensor to measure plunger position. Another employs a capacitive sensor with a patent-pending electrode design that does not contact the cartridge. Both TMR and capacitive sensing offer more reliable and accurate monitoring of injection volume, enhancing the precision and performance of drug delivery pens.
Microcontroller
A microprocessor or microcontroller is essential to control a drug delivery pen. Compact single-chip MCUs deliver all key functions, from monitoring plunger position to measuring the delivered volume, while keeping power use and size to a minimum. Sleep modes cut power consumption further during idle periods, making the device more efficient and long-lasting.
High-efficiency 8-bit MCUs are available for controlling pen operation. When all peripherals are inactive, some MCUs can operate at currents as low as five milliamperes, with lower clock speed settings reducing consumption even further. These MCUs have low-power inactivity modes that reduce current consumption to 0.7 µA.
Models can have 10 to 15 analog-digital converter input channels with 10-, 12-, or 14-bit resolution, 16 to 64 Kbyte flash memory, and an on-chip temperature sensor array. The MCUs have high-resolution timers for the PWM drive of motors. They also have peripheral I/O ports optimized for interfacing with wireless communication chipsets. Models can also have LCD drivers to control a display.
All this built-in capability can be housed in enclosures as small as a 32-QFN (5 mm x 5mm) package.
A handful of versatile, compact, and high-performance components make it possible to design the electronics for a fully functional drug delivery pen. These parts help engineers fit sophisticated circuitry into a small, user-friendly device, combining convenience with reliable performance.
Standards for drug delivery pens
Medical devices are highly regulated to ensure patient safety. Table 1 lists the standards with which design engineers must comply to develop a certified device. Understanding these standards and their impact on device design is essential for obtaining medical device certification from the regulating bodies.
Table 1. Applicable standards for drug delivery pens. Source: Littelfuse
| Standard |
Title |
General scope |
Market |
ISO
11608-1 |
Needle-based injection systems for medical use. Part 1: Needle-based injection systems |
Specifies requirements and test methods for Needle-Based Injection Systems (NISS) for single-patient use intended to deliver discrete volumes (bolus) of medicinal product, which can be delivered through needles or soft cannulas for intradermal, subcutaneous, and/or intramuscular delivery, or all three incorporating pre-filled or user-filled, replaceable, or non-replaceable containers. |
Global |
ISO
11608-4 |
Needle-based injection systems for medical use. Part 4: Needle-based injection systems containing electronics |
Specifies requirements and test methods for needle-based injection systems (NISS) containing electronics with or without software (NIS-Es) |
Global |
IEC
60601 |
Medical Electrical Equipment Testing and Certification |
Part 1-11: General requirements for basic safety and essential performance-Collateral standard Requirements for medical electrical equipment and medical electrical systems used in the home healthcare environment |
Global |
IEC
60529 |
Degrees of protection provided by enclosures (IP Code) |
Developed to rate and grade the resistance of enclosures of electrical and electronic devices against the intrusion of dust and liquids. It also rates how easy it is for individuals to access the potentially hazardous parts within the enclosure |
Global |
IEC
60068-2-XX |
Environmental testing of electrotechnical products |
A collection of methods for environmental testing of electronic equipment and products to assess their ability to perform under environmental conditions, including extreme cold and dry heat. |
Global |
Resources for efficient design
The surface-mount, low-power components highlighted in this article deliver the control needed while meeting strict size, power, and weight targets. By using the manufacturers' expertise, designers can choose cost-effective components more efficiently. Application engineers can guide selections, helping to reduce development time and keep costs under control.
Beyond assistance with component selection, Littelfuse engineers offer simulation and modeling services to optimize design performance with Littelfuse components. In addition, the application engineers can provide guidance on design requirements dictated by the applicable standards.
Littelfuse, amongst other manufacturers, can perform pre-compliance testing to avoid failures, saving testing costs and delays in introducing new products due to multiple compliance test cycles. By pairing some of the components suggested here with expert guidance, compact, low-power electronic drug delivery pens can be designed to meet performance and usability demands.
Reference literature
About the author
Dr. Marco Doms
Sr. Manager Technical Marketing, Littelfuse, Inc.

Marco studied electrical engineering and holds a PhD in MEMS. He was the head of R&D in two other sensor companies before joining Littelfuse in 2022. Marco has a long history in position sensors (especially xMR) and managing R&D and Innovation teams - from chip to system level.
At Littelfuse, he started as an Innovation Manager, led the EBU Advanced Development team, and introduced an Innovation/Idea Management process. In his current role, Marco is responsible for several platforms with entirely new products or product features that require additional internal and customer coordination.
About Littelfuse
Littelfuse is a diversified industrial technology manufacturing company empowering a sustainable, connected, and safer world. Across more than 20 countries, and with approximately 16,000 global associates, we partner with customers to design and deliver innovative, reliable solutions. Serving over 100,000 end customers, our products are found in a variety of industrial, transportation, and electronics end markets - everywhere, every day. Headquartered in Chicago, Illinois, United States, Littelfuse was founded in 1927.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.