Tropane alkaloids are toxic compounds predominantly found in plants belonging to the Solanaceae family, including deadly nightshade, henbane, mandrake, and Jimson weed.1 Atropine and scopolamine are the most prevalent tropane alkaloids detected in food samples.2 These alkaloids can contaminate herbal teas through plant debris or soil migration.3,4
Exposure to these alkaloids can induce symptoms such as reduced salivation, dry skin, and pupil dilation. At increased concentrations, they cause drowsiness, visual impairment, palpitations, disorientation, and hallucinations.5 The European Union (EU) has established a regulatory threshold of 0.2 ng/mL for the combined concentration of atropine and scopolamine in herbal infusions to reduce consumer risk.6
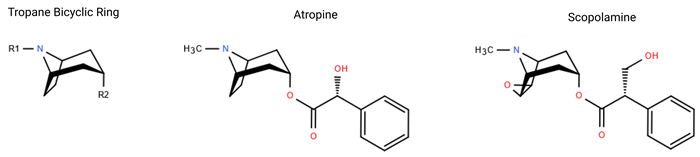
Figure 1. The chemical structures of tropane alkaloids include the tropane bicyclic ring structure, atropine, and scopolamine. Image Credit: Porvair Sciences Limited
Existing analytical methods for tropane alkaloid detection in food products employ solid-phase extraction (SPE) products with a large bed weight of over 150 mg. The usage of large solvents makes these methods time-consuming and less sustainable.1
This study verifies the extraction of atropine and scopolamine using a smaller 10 mg polymeric SCX 96-well SPE plate, followed by HPLC-MS/MS evaluation. After method validation, a selection of herbal teas available on the UK market, including chamomile, peppermint, and green tea, was analyzed to determine the existence of atropine and scopolamine in the final herbal tea infusions.
Materials and methods
Tea infusion, according to ISO 3103:1980:7
Two grams of tea leaves were added to a stainless-steel tea strainer inside a beaker, and 150 mL of boiling ultrapure water was then poured through the tea strainer. The resulting mixture was steeped for five minutes, cooled to ambient temperature, before being filtered through a 0.22 µm PES filter.
SPE procedure:
Sample processing employed a 10 mg 96-well plate (Microlute® CP 10 mg SCX) with a positive pressure manifold (UltraPPM™ Lite). Before being loaded onto the SPE plate, the pH of the tea infusion samples was adjusted with 0.1% formic acid (v/v).
SPE steps:
- Conditioning: 1 mL of methanol
- Equilibration: 1 mL of 0.1% formic acid in ultrapure water
- Loading: 1 mL of acidified tea infusion sample (0.1% formic acid)
- Wash 1: 1 mL of 0.1% formic acid in ultrapure water
- Wash 2: 1 mL of 0.1% formic acid in methanol
- Drying step: Dried at 20 PSI for 2 minutes using the positive pressure manifold
- Elution: 2 x 500 µL of 0.5% ammonia in methanol, dried at 20 PSI for 2 minutes following each elution
- Reconstitution: The eluate was evaporated at 30 °C with a nitrogen blowdown evaporator (Ultravap® Mistral) and reconstituted with 0.2 mL of 0.1% formic acid in ultrapure water
HPLC-MS/MS approach:
Table 1. The HPLC and Mass Spectrometer conditions used for analysis and the atropine and scopolamine MRM transitions. Source: Porvair Sciences Limited
| . |
. |
. |
. |
. |
. |
| HPLC: ACQUITY Premier UPLC |
Mass Spectrometer: Xevo TQ-S Micro |
| Solvent A H2O + 0.1% formic acid |
Needle Wash MeOH + 0.1% formic acid (three seconds) |
| Solvent B MeOH + 0.1% formic acid |
Syringe Draw Rate 30 µL/min |
Gradient
| Time (min) |
A (%) |
B (%) |
| 0.00 |
90.0 |
10.0 |
| 0.65 |
90.0 |
10.0 |
| 4.15 |
78.0 |
22.0 |
| 4.17 |
0.0 |
100.0 |
| 5.15 |
0.0 |
100.0 |
| 5.17 |
90.0 |
10.0 |
| 6.75 |
90.0 |
10.0 |
|
Needle Placement 4 mm |
| Injection Volume 5 µL |
| Column Waters ACQUITY UPLC-BEH C18 (2.1 x 50, 1.7 µm) |
| Column Temperature 30 °C |
| Source Type ESI |
| Capillary Voltage 1000 V |
| Desolvation Temperature 600 °C |
| Source Temperature 150 °C |
| Desolvation Gas Flow 1000 L/hr |
| Cone Gas Flow 0 L/hr |
| Flow Rate 400 µL/min |
Detector Gain 1.00 |
| Seal Wash On (five minutes) |
Minimum Points Per Peak 15 |
| Sample Temperature 13 °C |
|
| |
| MRM Transitions: |
| Analyte |
Precursor
ion (m/z) |
Product
ion (m/z) |
Cone
voltage (V) |
Collision
energy (V) |
| Atropine |
289.83 |
124.06 (Quan ion) |
30 |
22 |
| 92.95 |
30 |
| 90.99 |
42 |
| Scopolamine |
302.82 |
138.05 (Quan ion) |
28 |
18 |
| 156.03 |
15 |
| 102.94 |
40 |
Validation guidelines:
Testing was conducted in accordance with the pesticide validation guidelines for food (SANTE/11312/2021), and the Limit of Quantification (LOQ) was based on Regulation 2023/2783:8,9
Acceptance criteria:
- Recovery: 70-120% at three concentrations (0.2, 1.0, and 5.0 ng/mL)
- Repeatability: ≤20% at each level
- Linearity: R2 ≥0.99 with residuals ≤±20%
- LOQ: <0.05 ng/mL
Results and discussion
Method validation for SPE followed by HPLC-MS/MS
Table 2 presents the linearity, LOQ, and matrix effect (ME) for atropine and scopolamine in each validated herbal infusion.
Table 2. Linearity, LOQ, and matrix effects for chamomile, peppermint, and green tea infusions, following SPE and HPLC-MS/MS analysis. Source: Porvair Sciences Limited
Herbal
Infusion |
Linearity
(ng/mL) |
Atropine |
Scopolamine |
| R2 |
LOQ
(ng/mL) |
ME
(%) |
R2 |
LOQ
(ng/mL) |
ME
(%) |
| Chamomile |
0.5 - 50 |
0.992 |
0.010 |
-17 |
0.995 |
0.010 |
-38 |
| Peppermint |
0.5 - 50 |
0.996 |
0.010 |
-14 |
0.992 |
0.010 |
-24 |
| Green tea |
0.5 - 50 |
0.991 |
0.025 |
-12 |
0.993 |
0.025 |
-22 |
All herbal infusion matrices exhibited strong linearity in the 0.5 to 50 ng/mL range, with R2 values between 0.991 and 0.996. Furthermore, all calibration levels exhibited residuals of <±20%, indicating that the approach met the linearity specifications.
LOQ values for all herbal infusions were under the LOQ specification of 0.05 ng/mL. Chamomile and peppermint teas exhibited LOQ values of 0.010 ng/mL for both atropine and scopolamine.
Green tea demonstrated a slightly higher LOQ concentration of 0.025 ng/mL, attributed to baseline noise reducing the signal-to-noise ratio.
Matrix effect was also assessed for all three herbal infusions. All samples displayed a negative matrix effect (ion suppression), with atropine less affected than scopolamine.
Due to matrix effects exceeding ±20%, matrix-matched standards were employed for calibration during recovery and repeatability testing, following SANTE/11312/2021 guidance.
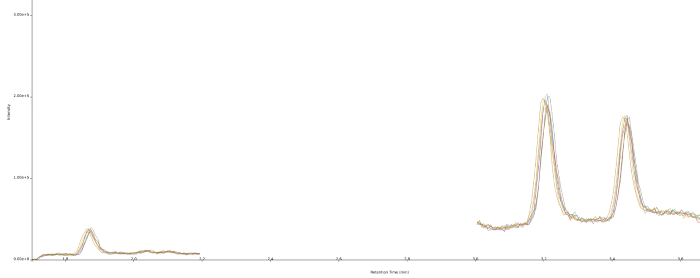
Figure 2. Chromatogram showing the LOQ of atropine and scopolamine at 0.010 ng/mL (n=8). Retention time is 1.85 min for scopolamine and 3.43 min for atropine. The resolution between atropine and the peak at 3.20 minutes is 5.0. Image Credit: Porvair Sciences Limited
Table 3. Recovery and repeatability data for chamomile, peppermint, and green tea infusions using the combined SPE and HPLC-MS/MS method for the analysis of atropine and scopolamine. Intra-day repeatability (n=8, in one day) and inter-day repeatability (n=16, on two days) SD = standard deviation. Source: Porvair Sciences Limited
| Herbal Infusion Sample |
Atropine |
Scopolamine |
| Recovery (% ± SD) |
Intra-day Repeatability (%RSD) |
Inter-day Repeatability (%RSD) |
Recovery (% ± SD) |
Intra-day Repeatability (%RSD) |
Inter-day Repeatability (%RSD) |
| Chamomile - 0.2 ng/mL |
93 ± 1.7 |
1.8 |
6.9 |
84 ± 3.1 |
3.6 |
4.8 |
| Chamomile - 1 ng/mL |
99 ± 1.6 |
1.6 |
9.8 |
94 ± 2.4 |
2.6 |
5.5 |
| Chamomile - 5 ng/mL |
98 ± 2.0 |
2.1 |
2.4 |
99 ± 2.1 |
2.2 |
2.5 |
| Peppermint - 0.2 ng/mL |
87 ± 0.88 |
1.0 |
2.4 |
85 ± 1.8 |
2.1 |
4.1 |
| Peppermint - 1 ng/mL |
95 ± 0.78 |
0.8 |
4.5 |
96 ± 1.6 |
1.7 |
5.7 |
| Peppermint - 5 ng/mL |
92 ± 1.6 |
1.7 |
2.2 |
95 ± 1.2 |
1.3 |
2.6 |
| Green Tea - 0.2 ng/mL |
78 ± 2.7 |
3.4 |
15.8 |
78 ± 2.5 |
3.2 |
10.8 |
| Green Tea - 1 ng/mL |
96 ± 1.2 |
1.3 |
5.1 |
93 ± 2.4 |
2.6 |
5.1 |
| Green Tea - 5 ng/mL |
96 ± 0.66 |
0.7 |
3.9 |
96 ± 1.4 |
1.5 |
2.1 |
Recovery and repeatability findings indicated satisfactory recovery across all herbal infusions at all concentrations analyzed, ranging from 78 – 99% for both atropine and scopolamine. Intra-day repeatability demonstrated exceptional results, ranging from 0.7 - 3.4 %RSD for atropine and 1.3 - 3.6 %RSD for scopolamine.
Inter-day repeatability was slightly higher, ranging from 2.2 - 15.8 %RSD for atropine and 2.1 - 10.8 %RSD for scopolamine. This reflects normal day-to-day variability and remains within specification. These results confirmed the method’s reliability for quantifying alkaloids in herbal infusions.
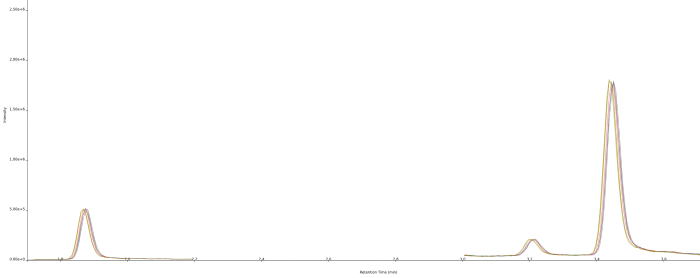
Figure 3. Overlaid chromatograms (n=8) showing the intra-day repeatability data for atropine and scopolamine in peppermint infusion at 0.2 ng/mL. Retention time is 1.85 min for scopolamine and 3.43 min for atropine. Image Credit: Porvair Sciences Limited
Table 4. Concentration values (± standard deviation, n=4) for atropine, scopolamine, and the sum of both analytes in herbal infusions prepared using the SPE and HPLC-MS/MS method. ND = Not detected, due to signal-to-noise ratio being less than 3:1. Source: Porvair Sciences Limited
Tea Infusion
Sample |
Concentration (ng/mL) |
| Atropine |
Scopolamine |
Sum of Atropine and Scopolamine |
| Chamomile-1 |
<LOQ |
ND |
<LOQ |
| Chamomile-2 |
<LOQ |
<LOQ |
<LOQ |
| Chamomile-3 |
0.013 ± 0.0035 |
<LOQ |
0.013 ± 0.0035 |
| Chamomile-4 |
<LOQ |
ND |
<LOQ |
| Chamomile-5 |
0.59 ± 0.0070 |
0.35 ± 0.012 |
0.94 ± 0.013 |
| Chamomile-6 |
<LOQ |
ND |
<LOQ |
| Peppermint-1 |
<LOQ |
<LOQ |
<LOQ |
| Peppermint-2 |
<LOQ |
<LOQ |
<LOQ |
| Peppermint-3 |
<LOQ |
0.011 ± 0.0014 |
0.011 ± 0.0014 |
| Green tea-1 |
<LOQ |
ND |
<LOQ |
| Green tea-2 |
ND |
ND |
ND |
| Green tea-3 |
<LOQ |
ND |
<LOQ |
Twelve herbal tea infusions of chamomile, peppermint, and green tea were analyzed in quadruplicate using the validated SPE and HPLC-MS/MS method. Most samples exhibited low or undetectable concentrations of atropine and scopolamine.
However, one chamomile sample (chamomile-5) notably exceeded EU regulatory limits, with a concentration of 0.59 ng/mL atropine and 0.35 ng/mL scopolamine, resulting in a combined concentration of 0.94 ng/mL, approximately five times the EU limit.
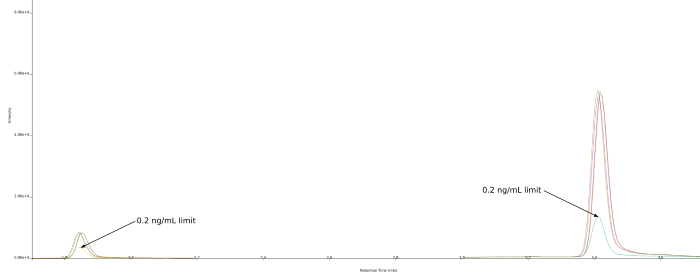
Figure 4. Overlaid chromatograms showing the 0.2 ng/mL EU limit reference standard versus chamomile-5’s chromatograms (n=4) at a mean concentration of 0.35 ng/mL for scopolamine and 0.59 ng/mL for atropine. Retention time is 1.85 min for scopolamine and 3.43 min for atropine. Image Credit: Porvair Sciences Limited
Conclusions
The study verified a solid-phase extraction approach utilizing a reduced bed weight (10 mg) SCX resin in a 96-well format for atropine and scopolamine analysis in widely consumed herbal infusions.
The technique complied with SANTE/11312/2021 validation requirements, demonstrating strong linearity, low LOQs, and high recovery with consistent repeatability both intra- and inter-day, across three concentrations (0.2, 1.0, and 5.0 ng/mL).
When applied to commercial products, most infusions contained low or undetectable concentrations of atropine and scopolamine. One chamomile sample, however, surpassed the regulatory threshold for both atropine and scopolamine by nearly fivefold.
These findings indicate that a low-bed-weight SPE approach is adequate for analyzing herbal tea samples and can provide a more sustainable alternative than higher-bed-weight SPE approaches.
Want to learn more?
Join Porvair's webinar on the 25th November 2025.

Morning session:
10:00 AM - 11:00 AM (GMT)
Afternoon session:
4:00 PM - 5:00 PM (GMT)
Acknowledgments
This article is based on materials originally published in International Labmate, volume 50, issue 5.
References and further reading:
- González-Gómez, L., et al. (2022). Occurrence and Chemistry of Tropane Alkaloids in Foods, with a Focus on Sample Analysis Methods: A Review on Recent Trends and Technological Advances. Foods, 11(3), p.407. https://doi.org/10.3390/foods11030407.
- Mulder, P.P.J., et al. (2016). Occurrence of tropane alkaloids in food. EFSA Supporting Publications, 13(12). https://doi.org/10.2903/sp.efsa.2016.en-1140.
- Monique de Nijs, Crews, C., et al. (2023). Emerging Issues on Tropane Alkaloid Contamination of Food in Europe. 15(2), pp.98–98. https://doi.org/10.3390/toxins15020098.
- González-Gómez, L., et al. (2022). Improved Analytical Approach for Determination of Tropane Alkaloids in Leafy Vegetables Based on µ-QuEChERS Combined with HPLC-MS/MS. Toxins, (online) 14(10), p.650. https://doi.org/10.3390/toxins14100650.
- AGES. (2023). Tropane alkaloids. (online) Available at: https://www.ages.at/en/human/nutrition-food/residues-contaminants-from-a-to-z/tropane-alkaloids.
- Commission Regulation (EU). (2023). EUR-Lex - 02023R0915-20250101 - EN - EUR-Lex. (online) Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02023R0915-20250101.
- ISO 3103: Tea - Preparation of liquor for use in sensory tests, 1980 edition.
- Main changes introduced in Document No SANTE/11312/2021v2 with respect to the previous version (Document No SANTE/11312/2021). Available at: https://food.ec.europa.eu/system/files/2023-11/pesticides_mrl_guidelines_wrkdoc_2021-11312.pdf.
- Commission Implementing Regulation (EU) (2023). Implementing regulation - EU - 2023/2783 - EN - EUR-Lex. [online] Available at: https://eur-lex.europa.eu/eli/reg_impl/2023/2783/oj.
About Porvair Sciences Limited

Porvair Sciences
Porvair Sciences are global leaders in the manufacturing and development of cutting-edge porous plastic materials and microplate technologies for the biotechnology and life science industries. From microplates and assay kits to automated laboratory equipment, our company is committed to the creation of workflow solutions with high quality products for improved analysis. Offering customers a diverse portfolio dedicated to high quality sample preparation, our innovative products are designed to increase productivity and accelerate scientific discovery with integrity.
Microlute®
The Microlute® range of sample preparation products were designed with reproducibility in mind. At the heart of the range are products with a unique hybrid structure, comprising a porous plastic disc with embedded active materials to enhance sample cleanup and analyte recovery. Discover the range of sample preparation solutions including solid phase extraction (SPE), phospholipid removal (PLR) and supported liquid extraction (SLE).
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.