Precise water analysis is essential in ensuring pharmaceutical products’ performance, safety, and adherence to regulations.
Karl Fischer (KF) titration is a widely used method for water content determination due to its high selectivity to water molecules. KF titration involves the reaction between sulfur dioxide, iodine, and water molecules in an alcohol solvent and base.
The current or potential measured between pins is often used as a monitoring tool for the Karl Fischer reaction, but the chemical reaction of iodine consumption can also be used to quantify the results.
Two primary types of KF titration exist:
- Volumetric method
- Coulometric method
The best-suited method will depend on the sample matrix under investigation and the sample’s water content.
Both the European Pharmacopoeia (Ph.Eur.) and the United States Pharmacopeia (USP) have established guidelines and regulations relating to KF titration, with these regulations helping to ensure that methods employed in water content determination are standardized, reliable, comparable, and acceptable across pharmaceutical industries.
This article explores the use of volumetric KF titration in the measurement of pharmaceutical samples’ water content, ranging from 100 ppm to 100 %. The measurements are performed in accordance with USP and Ph. Eur. guidance.

Image Credit: Mettler-Toledo - Titration
USP 921 and Ph. Eur. 2.5.12 regulations
Water content determination using KF titration
These guidelines and regulations are designed to ensure that the methods employed in determining water content are standardized and reliable.
The USP 921 specifies two methods:
- Direct titration (Method Ia)
- Residual Titration (Method Ib)
USP 921. Source: Mettler Toledo - Titration
| Method Ia |
Method Ib |
| Direct Titration |
Residual Titration |
| Involves adding an appropriate amount of KF reagent to the sample until the water completely reacts and the endpoint is reached. |
Involves adding excess KF reagent to the sample to ensure that all water is reacted, followed by the back-titration of the excess reagent with a standard solution of KF reagent. |
| The amount of water in the sample is calculated from the amount of KF reagent used. |
The amount of water in the sample is calculated from the amount of excess KF reagent used in the back-titration. |
Ph. Eur. 2.5.12 specifies two methods:
Ph. Eur. 2.5.12. Source: Mettler Toledo - Titration
| Method A |
Method B |
| Direct Titration |
Back Titration |
| Directly titrates the sample using a suitable KF reagent and an appropriate indicator to reach the endpoint. |
Involves adding excess KF reagent to the sample, followed by the back-titration of the excess reagent with a standard solution of KF reagent. |
| The amount of water in the sample is calculated from the volume of the KF reagent used. |
The amount of water in the sample is calculated from the amount of excess KF reagent used in the back-titration. |
Volumetric Karl Fischer titration process
It is necessary to follow a few important steps to ensure the accuracy and reliability of titration analysis in the laboratory.
Preparation: System setup
Preparation is a key step in any process, including titration. This involves choosing the right reagents, determining method parameters, and ensuring all equipment is clean and impurity-free.
Eliminate water: Cell conditioning
Pre-titration (conditioning) is an essential step in ensuring accurate results in KF titration because it ensures the titration system is in a dry state. Pre-titration removes water from the inner surface of the titration vessel and the solvent (reagent).
Standardization: Titer determination
Another critical step in the titration process, standardization, involves determining the precise concentration of the titrant via titer determination. This allows the correction of any deviations resulting from the equipment or environment that could potentially impact the accuracy of the water content measurements.
Process check: Control with standards
The combination of a process check and standardization steps ensures accurate sample analysis. A process check is essentially a quality control measure that requires the periodic analysis of known reference samples to ensure measurement accuracy and reliability.
Water content: Sample titration
The sample preparation process involves ensuring the full release of water from the sample by:
- Adding co-solvents to enhance solubility
- Applying heat to enable mixing
- Using a homogenizer to ensure consistent sample distribution
Once sample preparation is complete, it is possible to titrate the water released from the sample matrix.
Key components of a KF titrator
The titrant contains the iodine for the reaction in volumetric Karl Fischer titrations. The titrator controls and measures the volume of titrant added to the Karl Fischer cell.
Titrant volume and concentration are used to calculate the amount of water removed from the Karl Fischer cell. The titrator measures the titrant volume during the titration and uses the titrant concentration stored on the titrator to calculate the sample’s water content.
Burette
The burette and dosing unit aspirate the titrant from the titrant bottle before dispensing titrant into the Karl Fischer cell.
Karl Fischer cell
The Karl Fischer cell is comprised of an assembled titration vessel used during the reaction. Moisture from the surrounding air may react with the iodine in the solution; however, this may lead to inaccurate results.
It is important to maintain a dry environment to ensure accurate measurements. This can be achieved by taking necessary precautions to protect the solvents and reagents from atmospheric moisture.
Sensor
The double-pin platinum electrode is used to determine the endpoint of the reaction by measuring the electrical current.
Stirrer
Thoroughly mixing the sample, titrant, and solvent in the vessel is essential to ensure an accurate measurement. A stirrer facilitates this process, promoting an even distribution of substances, increasing the likelihood of reactants meeting, and minimizing the accumulation of iodine ions around the sensor.
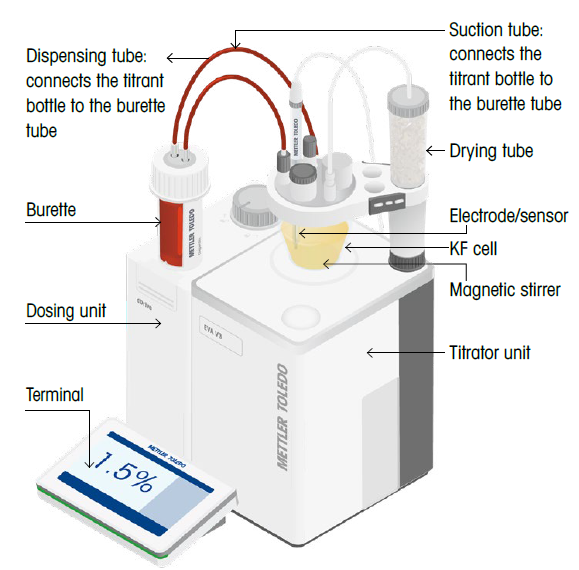
Image Credit: Mettler Toledo - Titration
Reagents required for analysis
Reagents employed in Karl Fischer titration may be either one-component or two-component.
The choice between using one-component or two-component reagents in Karl Fischer titration is based on user preference. Two-component reagents are more stable than one-component reagents, though one-component reagents tend to be less expensive.
One-component reagents
One-component reagents are composed of a titrant containing sulfur dioxide, iodine, and imidazole dissolved in an appropriate alcohol. The solvent used is generally dry methanol.
Two-component reagents
Two-component reagents are composed of a titrant containing iodine and methanol, while the solvent used includes imidazole, sulfur dioxide, and methanol (MeOH).
Traditional reagents like pyridine and methanol continue to be used, but there is an increasing trend towards using safer alternatives such as MeOH-free reagents that are not carcinogenic, mutagenic, and reprotoxic (CMR).
Source: Mettler Toledo - Titration
| |
USP 921 |
Ph.Eur. 2.5.12 |
| Preparation of reagents |
It actually provides the preparation of reagents with pyridine and methanol Not more than 2 mg/ ml water when determining trace amounts of water below one percent. |
It does not describe how the reagents are being prepared. |
| Recommended titrant composition |
Stabilized reagents without pyridine, such as one or two-component reagents or alcohol other than methanol, such as diethylene glycol monoethyl ether. |
It describes that water reacts with a reagent that contains a suitable anhydrous medium, such as alcohol, a base with sufficient buffering capacity, sulfur dioxide, and iodine. |
Sample preparation
Ensuring complete water release from the sample matrix
Technique selection typically depends on the nature of the sample being analyzed. The table below lists a range of techniques and the types of samples they are suitable for use with.
Source: Mettler Toledo - Titration
| Technique |
Nature of Sample |
Preparation Method |
Comments |
Direct
Addition |
- Soluble
- No side reactions
|
No specific sample preparation
If required
Adjust pH value. Improve solubility
- Auxiliary solvents
- Warming/heating
|
If the sample has high solubility, it can be dissolved directly in the Karl Fischer solvent.
For example, antibiotics with low solubility in a sample can be challenging to analyze. It is recommended to use a Thermovessel set at 50 °C to improve solubility and facilitate the analysis.
|
| Internal Extraction |
- Insoluble
- No side reactions
- Fast water release
|
Extraction by :
- Long stir time (5-10 min)
- High-speed mixer Improve solubility
- Aux. solvents
|
A homogenizer is used for mechanically crushing the sample in the titration vessel, for instance, grinding tablets like aspirin. |
| External Extraction |
- Insoluble
- Slow water release
- Very low water content
|
Extraction by :
- Suitable solvent or solvent mixture
|
External extraction involves the use of a compatible solvent to extract water from the sample matrix. |
| External Dissolution |
- Soluble
- Non-homogenous water distribution
- Very low water content
|
Dissolution by :
- Suitable solvent or solvent mixture
|
Dissolution utilizes special reagents like co-solvents for APIs that contain ketone and aldehyde groups, buffers for acidic components, and formamide for sugar coated APIs. |
| Gas-phase Extraction |
- Side reactions with KF reagents
- Insoluble
- Slow water release
|
After heating and collecting the vapor, the water containing gas will come into contact with the solvent or titrant when transferred into the titration cell.
Heating time: min 10 min, max 30 min
|
The dry air carries the water vapor from the sample and transfers it into the titration cell. |
System requirements and initial checks
The accuracy and precision of any titration system can be affected by several factors.
Drying unit
It is important to apply a suitable drying unit material to the titration vessel, solvent bottle, titrant bottle, and waste bottle. Silica gel particles in molecular sieves can be used for color coding, with a color change indicating that the sieve needs to be replaced.
Flat seals should be placed on the top of all bottles, including solvent, titrant, and waste. This is key to reducing water ingress from the environment.
Instrument calibration
The instrument should be regularly calibrated and maintained to ensure it continues to function properly.
Electrode maintenance
The instrument’s platinum pins should be cleaned by wiping them with tissue or by soaking them in 50 % HNO3 for 10 minutes prior to rinsing with deionized water and dry methanol. Pins should be submerged in methanol in the titration beaker.
Laboratory temperature and humidity
Lab temperature and humidity should be controlled and maintained within a specific range to prevent changes in the chemical reaction rate and ensure accurate results. This is important because moisture can interfere with the Karl Fischer reagents, adversely impacting the accuracy of results.
Image Credit: Mettler Toledo - Titration
Standards for titer determination
Water standard
It is advisable to use a water standard one percent certified reference material for titer determination, with one gram typically corresponding to approximately 10 mg of water, which is the ideal amount for volumetric KF titration.
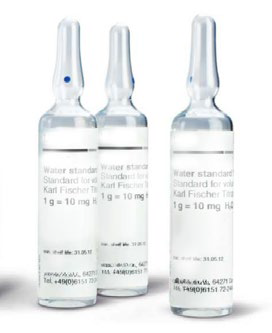
Image Credit: Mettler Toledo - Titration
Di-sodium tartrate dihydrate
Di-sodium tartrate dihydrate can be used as a standard for titer determination, but it has limited solubility in methanol. This means that prolonged stirring periods may be required, extending the time required. Di-sodium tartrate dihydrate also saturates after approximately 150 mg.

Image Credit: Mettler Toledo - Titration
Pure water
Pure water is an easily accessible standard for titer determination; however, its low sample weight of 10-30 µL can lead to high weighing failure rates and challenging sample handling.

Image Credit: Mettler Toledo - Titration
Eliminating residual water with conditioning
Importance of the drift value
Titrant consumption is measured in µg/mL or µL/mL. Drift is only linked to physical drift under ideal circumstances, but it can also be linked to chemical drift where there is a risk of side reactions. Drift would increase from sample to sample in this instance.
Sources of residual water can affect the physical drift value linked to the titration cell, including:
- Traces of water in the solvent
- Surface water in the titration vessel
- Atmospheric humidity entering the titration cell
It is possible to significantly reduce these sources, but they cannot be eliminated entirely. However, the whole cell can reach a dry state in which the drift value is below a certain threshold value. This is important because maintaining low, constant drift is key to ensuring precise results.
It is also important to determine the drift of the titration stand before commencing the titration process.

Image Credit: Mettler Toledo - Titration
Pre-titration (conditioning) and standardization
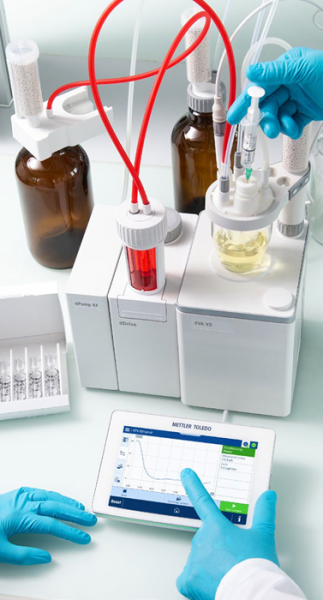
Image Credit: Mettler Toledo - Titration
Source: Mettler Toledo - Titration
| |
USP 921 |
Ph. Eur. 2.5.12 |
| Pre-titration |
Place methanol or a suitable solvent in the titration cell to cover the electrode. |
Add methanol or another suitable solvent. |
| Eliminate any residual water from the titration cell. Add reagent (titrant) until the endpoint color is reached or the appropriate electrode response. |
Eliminate any residual water from the titration cell and solvent. |
Standardization
(titer determination) |
Standardize the reagent by adding commercial standards with a Certificate of Analysis (CoA) traceable to a national standard or sodium tartrate. |
Add a certified reference material, such as a water standard, or purified water, while stirring for the necessary time. |
| When freshly prepared, 1 mL of this solution can be equivalent to approximately 5 mg (most commonly used) or 2 mg or 1 mg of water, but it deteriorates gradually. Therefore, standardize it within one hour before use or daily if in continuous use. |
Ensure the titrant concentration is at least 80 % of the value specified in the supplier's Certificate of Analysis. Standardize after opening the bottle for the first time and at regular intervals thereafter. |
USP 921: Suitability test
Test preparation and titration
It is important to follow specific instructions when preparing a sample for water content analysis, depending on the type of sample under investigation.
When working with aerosol samples with propellant, storing the container in a freezer for at least two hours before opening and testing is necessary. The endpoint should be determined at a temperature of 10 °C or higher.
When working with capsule and tablet samples, using a mixed portion of at least four tablets or capsules ground to a fine powder is advisable.
Hygroscopic specimens require introducing a precisely weighed portion of the solid into the titration vessel as soon as possible to avoid moisture uptake from the atmosphere.
When dissolving the sample, it is important to accurately measure a suitable solvent (such as methanol) and a dry syringe. Water content should be determined in milligrams before being subtracted from the total water content obtained in the titration of the specimen being tested.
Care should be taken to avoid dehydration due to decomposition of the sample constituents when determining the weight of a specimen tested, where external heat is used to release water from the sample. It is also important to consider and correct any drift due to the transport gas.

Image Credit: Mettler Toledo - Titration
Ph. Eur. 2.5.12: Suitability test
Preparation, titration, and result interpretation
The Ph. Eur. 2.5.12. includes a linearity check with acceptance criteria. The outcome of this check is the linearity, including x- and y-errors (e1 and e2).
A series of titrations must be performed on each combination of substance, titrant, and solvent to be examined to verify titrant accuracy.
The sample substance should be added to the titration vessel before the exact water content is titrated using the chosen titrant.
Five sequential known amounts of water should be added to the vessel, corresponding to approximately 50 % to 100 % of the water amount in the substance being tested.
Additional titrations should be performed after adding each known amount of water to verify the titrant's accuracy and the titration method’s overall consistency.
It is advisable to limit analysis to a linear range that enables the correct recovery of added quantities of water. This will help ensure accurate results in the presence of non-linearity and errors e1 and e2, because this approach can overcome the challenge of immediately correcting these errors.
The slope (b) and the percentage errors of the x- and y-axis intercepts (e1 and e2) are calculated on plotting the cumulative water added versus the sum of the initial amount and the cumulative water after each addition.
In this instance, e1 and e2 are the errors in percentage relative to the initially present water in the sample.
Acceptance criteria for b, e1, and e2 are as follows:
- b should be between 0.975 and 1.025
- e1 and e2 should be less than 2.5 %.
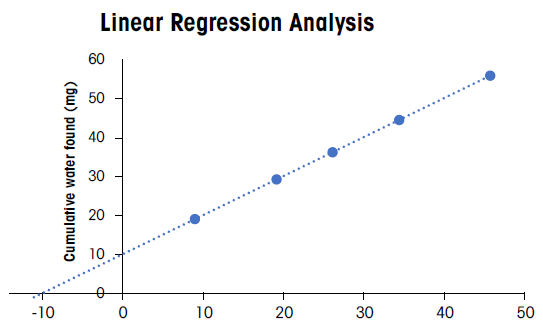
Image Credit: Mettler Toledo - Titration
Water determination under USP 921

Image Credit: Mettler Toledo - Titration
Source: Mettler Toledo - Titration
| Method Ia |
Method Ib |
| Direct Titration |
Residual Titration |
| The endpoint is determined electrometrically with an apparatus employing a simple electrical circuit that serves to impress about 200 mV of applied potential between a pair of platinum electrodes immersed in the solution to be titrated. |
Quickly add the test preparation, mix, and add an accurately measured excess of the reagent. Allow sufficient time for the reaction to reach completion, and titrate the unconsumed reagent with a standardized water solution to the electrometric or visual endpoint. Calculate the water content of the specimen. |
| At the endpoint of the titration, a slight excess of the reagent increases the flow of current to between 50 and 150 microamperes for 30 seconds to 30 minutes, depending upon the solution being titrated. The time is shortest for substances that dissolve in the reagent. |
Titrate the unconsumed reagent with a standardized water solution to the visual or electrometric endpoint and then calculate the specimen's water content. |
Water determination under Ph. Eur. 2.5.12

Image Credit: Mettler Toledo - Titration
Source: Mettler Toledo - Titration
| Method A |
Method B |
| Direct Titration |
Back Titration |
| Introduce methanol R into the titration vessel, or the solvent indicated in the monograph or recommended by the supplier of the titrant. Where applicable for the apparatus used, eliminate residual water from the measurement cell or carry out a pre-titration. Introduce the substance to be examined rapidly and carry out the titration, stirring for the necessary extraction time. |
Introduce methanol R into the titration vessel, or the solvent indicated in the monograph or recommended by the supplier of the titrant. Where applicable for the apparatus used, eliminate residual water from the measurement cell or carry out a pre-titration. Introduce the substance to be examined rapidly and in a suitable state of division. Add an accurately measured volume of the titrant, sufficient to give an excess of about 1 mL or the prescribed volume. Allow to stand protected from light for 1 min or the prescribed time, with stirring. |
| The end-point is identified by using two identical indicator electrodes that are connected to an electrical source and maintain a constant current or voltage. The titrant causes either a decrease in voltage (constant current) or an increase in current (constant voltage) until the end-point is reached. |
Titrate the excess of reagent using methanol R or the prescribed solvent, containing an accurately known quantity of water. |
The ideal titrator for volumetric KF titrations
The EVA volumetric Karl Fischer titrator is an efficient, reliable solution for the determination of the water content of liquids and solids. This titrator supports a variety of sample types, including insoluble solids, and is available in two models, the EVA V1 and EVA V3.
Precision and efficiency
The Fast Forecasting Amperometric (FFA™) algorithm speeds up reaction rates by optimizing reagent concentration. The titrator’s high-resolution dDrive unit ensures precise titrant addition, while its digital sensor captures accurate data.
Optimal safety
The instrument’s advanced solvent management system has been designed to automate chemical dispensing, reduce exposure, and help users maintain a safer, cleaner lab environment. Safe and remote operation is enabled via a detachable touchscreen and OneClick™ interface.
Regulatory compliance and traceability
The EVA Karl Fischer titrator has been designed to seamlessly integrate with LabX™ software in order to improve data management and adhere to key standards such as 21 CFR Part 11 and EU Annex 11.
Features such as electronic signatures, centralized user management, and comprehensive audit trails ensure adherence to ALCOA++ principles while enhancing data integrity and streamlining validation and maintenance.

Image Credit: Mettler Toledo - Titration
Mettler Toledo’s services
Mettler Toledo supports and services the company’s range of measurement equipment through its entire life cycle, including installation, calibration, preventive maintenance, and equipment repair.
Benefits of this working relationship include:
- Consistent equipment uptime, including a repair service, software care, provision of spare parts and kits, and service support
- Performance maintenance and optimization, including calibration, certification, and equipment qualification
- Compliance, ensuring calibration and quality through professional installation and preventive maintenance
- Expertise, including consulting, user training, access to documentation and downloads, and advice on good titration practice
Acknowledgments
Produced from materials originally authored by Mettler Toledo.
About Mettler Toledo - Titration
METTLER TOLEDO instruments are used in research, scientific, and quality control labs, amongst many others in the pharmaceutical, chemical, food, and cosmetics industries. They are a global market leader with the three instrument groups most frequently used in the laboratory, like balances, pipettes, and pH meters, and we are a pioneer in the field of Automated Chemistry.
METTLER TOLEDO industrial solutions cover the diverse steps in a host of manufacturing processes at many of the same customers as served for the laboratory. Solutions range from receiving raw materials, in-line process control, and end-of-line packaging control, to logistics and shipping. Increasingly, these solutions are fully integrated into the customer's IT environment, helping automate their workflows.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.