Alzheimer’s disease (AD) diagnosis is changing fast, driven by the need for earlier, more reliable, and easier-to-access detection methods.
Image Credit: Kateryna Kon/Shutterstock.com
With the recent approval of monoclonal antibody therapies such as lecanemab (tradename: Leqembi®) and donanemab (Kisunla™), the clinical imperative for accurate preclinical and early-stage AD diagnosis has intensified.
Traditional diagnostic modalities - cerebrospinal fluid (CSF) neurochemical dementia diagnostics and amyloid-PET imaging - are effective but impractical for large-scale screening due to invasiveness, cost, and logistical constraints. This has catalyzed the development of blood-based biomarkers, which promise to revolutionize early AD detection and patient stratification for preventive treatment.
A key technological advance in this domain is the use of immunoprecipitation (IP) as a preparatory step before high-sensitivity immunoassays (IA), collectively termed IP-IA. This approach enhances the detection of low-abundance biomarkers and improves diagnostic accuracy by enriching target proteins and clearing interfering matrix components.
This review evaluates the clinical relevance, methodological innovations, and diagnostic performance of IP-IA platforms for blood-based AD biomarkers, with a focus on their application in early and preclinical disease stages.
Clinical context and the need for blood-based biomarkers
The approval of disease-modifying monoclonal antibodies for early AD has shifted the diagnostic paradigm toward identifying patients in the earliest stages of disease - before significant cognitive decline and irreversible neurodegeneration occur.
The AD continuum, as defined by Jack et al., spans from preclinical stages (0–3) to clinical dementia (stages 4-6). Early preclinical AD (stages 1-2) is characterized by subtle molecular changes without overt symptoms, while early symptomatic AD (stage 3 and early dementia) presents with mild cognitive impairment (MCI) and functional decline.
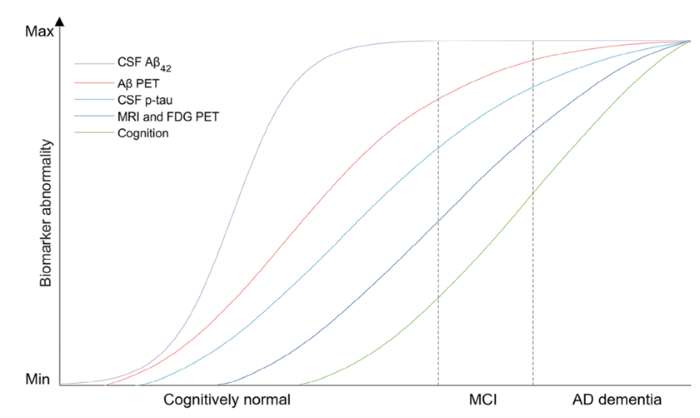
Image Credit: Lucy A., et al. Alzheimer´s Dement. 2025; 21:e14528. https://doi.org/10.1002/alz.14528
Current gold-standard diagnostics, such as CSF biomarker analysis and amyloid-PET, are unsuitable for population-level screening. Blood-based biomarkers, particularly those reflecting amyloid-beta (Aβ) and tau pathology, are emerging as practical alternatives. However, the challenge lies in achieving sufficient sensitivity and specificity, especially in the earliest disease stages.
Biomarker dynamics across the AD continuum
The earliest detectable biomarker change in AD is a decrease in CSF Aβ42, specifically the Aβ42/Aβ40 ratio. This change is mirrored in blood, occurring concurrently with CSF alterations. Other biomarkers, such as phospho-tau isoforms (notably p-tau217 and p-tau181), rise later in the disease course. Importantly, some patients with subjective cognitive deficits or early MCI may not show pathological amyloid-PET or tau-PET findings, underscoring the need for sensitive blood-based assays.
Methodological innovation: The IP-IA platform
The IP-IA methodology combines robotic IP with high-throughput immunoassays. The initial IP step uses functionalized magnetic beads with antibodies targeting diagnostic proteins (Aβ peptides, tau proteins), enriching the biomarkers and removing matrix components that can mask epitopes or interfere with detection.
A novel dilution technique, which involves heat denaturation and non-charged detergents, further enhances assay performance by exploiting the physicochemical properties of Aβ and tau proteins.
Following IP, eluates are analyzed using commercial immunoassay platforms (e.g., ELISA, MesoScale, Simoa, Lumipulse, Elecsys), enabling multiplexed detection of Aβ and tau epitopes from minimal plasma volumes (as little as 100 microliters). This is particularly advantageous for large cohort studies and precious sample sets.
Diagnostic performance and use cases
Multiple studies have demonstrated that IP-IA significantly improves the diagnostic accuracy of blood-based Aβ and tau measurements compared to direct assays.
For example, in collaboration with Roche Diagnostics, prior IP increased the area under the curve (AUC) for Aβ42/Aβ40 ratio detection from 0.73 to 0.88, and further to 0.92 with biomarker-supported clinical diagnosis.
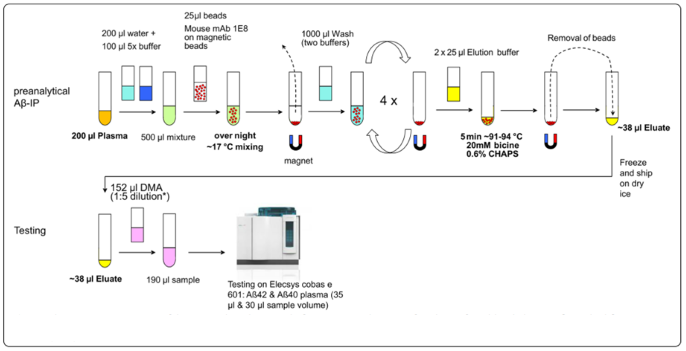
Fig 1. Schematic representation of the pre-analytical process before automated testing of IP-eluates from blood plasma. Aβ, amyloid-β; IP, Immunoprecipitation. Image Credit: Klafki, H. W., et al. (2020). International Journal of Molecular Sciences, 21
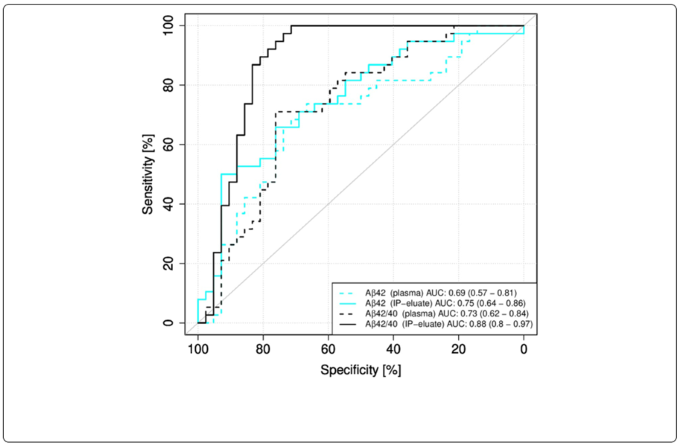
Fig 3. Receiver operating characteristic (ROC) curves for Elecsys measurements without or with pre-analytical immunoprecipitation (IP). ROC curves for the classification of the study participants into the diagnostic groups Aβ-positive (Aβ+) and Aβ-negative (Aβ-) were calculated for Elecsys measurements in plasma or IP-eluates. 95 % confidence intervals were calculated using the DeLong approach and are indicated in brackets. Aβ, amyloid-β: Aβ+, Aβ-positive: CSF Aβ42/40 ≤ 0.050; Aβ-, Aβ-negative: CSF Aβ42/40 > 0.050; IP, Immunoprecipitation. Image Credit: Klafki, H. W., et al. (2020). International Journal of Molecular Sciences, 21
Similar improvements were observed with the Lumipulse platform, where IP enhanced phospho-tau181 detection accuracy.
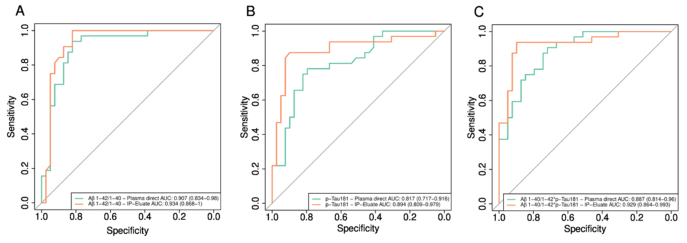
Fig 5. Pairwise comparisons of single value ROC curves for plasma biomarkers measured directly or after pre-analytical IPs on the Lumi-pulse platform. ROC curves for the discrimination between amyloid-positive and amyloid-negative study participants were calculated for A Aβ1-42/1-40, B pTau181, and C Aβ1-40/Aβ1-42 * pTau181. Aβ amyloid-β. IP immunoprecipitation. Image Credit: Morgado, B., Wiltfang, J. (2024). European Archives of Psychiatry and Clinical Neuroscience. Advance online publication. https://doi.org/10.1007/s00406-023-01751-2
Composite biomarker scores, such as the AT term (combining Aβ ratio and phospho-tau levels), further boost diagnostic performance. In large multicenter cohorts (e.g., the German DZNE Delcode study), logistic regression models using IP-IA data achieved AUCs up to 0.97 for CSF-validated AD diagnosis, with sensitivity and specificity exceeding 90 %.
These assays can reliably identify cognitively unimpaired individuals at high risk (Jack stage 1), decades before clinical dementia onset.
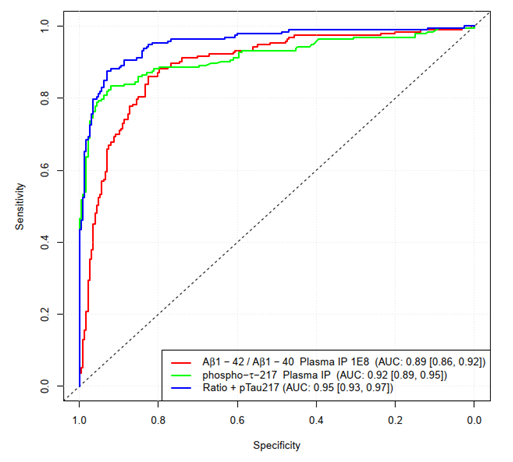
Figure 3.6. ROC curves for the prediction of CSF amyloid positivity. Classification was done using a logistic regression with leave-10-out cross-validation. Classes BM+ and BM- were defined using the CSF Aβ42/40 ratio with a threshold of 0.08. Image Credit: Morgado, B. et al. unpublished results; multicenter DELCODE1 cohort of the German Center for Neurodegenerative Diseases (DZNE)
Statistical analyses using standardized effect sizes (Cohen’s d, Cliff’s delta) confirm that IP-IA platforms provide robust discrimination between early preclinical AD and controls, with effect sizes and diagnostic accuracy comparable to or exceeding those of direct measurement, especially for phospho-tau217.
Multiparametric approaches and biological insights
Recent research highlights the importance of multiparametric biomarker strategies. While phospho-tau217 is a highly specific marker for early symptomatic AD, its sensitivity in preclinical stages is comparable to the Aβ42/Aβ40 ratio. Moreover, elevations in phospho-tau217 may arise from non-brain sources (e.g., muscle in ALS), suggesting that a combination of Aβ and tau markers is necessary for reliable early diagnosis.
The use of dual cut-off strategies (defining gray zones for further testing) and composite scores (e.g., AT term) enables nuanced patient stratification and minimizes diagnostic uncertainty. These approaches are critical for clinical decision-making, especially as blood-based assays move toward routine use.
Conclusion
Immunoprecipitation-enhanced immunoassay platforms (IP-IA) represent a major advance in the quest for accessible, accurate, and early diagnosis of Alzheimer’s disease. By enriching target biomarkers and clearing matrix interferences, IP-IA enables sensitive detection of Aβ and tau proteins from minimal blood volumes, facilitating large-scale screening and longitudinal studies.
Literature:
- Abu-Rumeileh, S., et al. (2025). Phosphorylated tau 181 and 217 are elevated in serum and muscle of patients with amyotrophic lateral sclerosis. Nature Communications, 16(1). DOI: 10.1038/s41467-025-57144-7. https://www.nature.com/articles/s41467-025-57144-7.
- Gonzalez-Ortiz, F., et al. (2022). Brain-derived tau: a novel blood-based biomarker for Alzheimer’s disease-type neurodegeneration. Brain, 146(3). DOI: 10.1093/brain/awac407. https://academic.oup.com/brain/article/146/3/1152/6960988?login=false.
- Hu, Y., et al. (2022). Assessment of a Plasma Amyloid Probability Score to Estimate Amyloid Positron Emission Tomography Findings Among Adults With Cognitive Impairment. JAMA network open, 5(4), pp.e228392–e228392. DOI: 10.1001/jamanetworkopen.2022.8392. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2791438.
- Jack, C.R., et al. (2024). Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimer’s & dementia, 20(8). DOI: 10.1002/alz.13859. https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/alz.13859,
- Janelidze, S., et al. (2021). Head-to-Head Comparison of 8 Plasma Amyloid-β 42/40 Assays in Alzheimer Disease. JAMA Neurology, (online) 78(11), pp.1375–1382. DOI: 10.1001/jamaneurol.2021.3180. https://jamanetwork.com/journals/jamaneurology/fullarticle/2784411.
- Jessen, F., et al. (2018). Design and first baseline data of the DZNE multicenter observational study on predementia Alzheimer’s disease (DELCODE). Alzheimer’s Research & Therapy, 10(1). DOI: 10.1186/s13195-017-0314-2. https://link.springer.com/article/10.1186/s13195-017-0314-2.
- Klafki, H.W., et al. (2020). Development and Technical Validation of an Immunoassay for the Detection of APP669–711 (Aβ−3–40) in Biological Samples. International Journal of Molecular Sciences, 21(18), p.6564. DOI: 10.3390/ijms21186564. https://pub.dzne.de/record/154067/files/DZNE-2021-00046.pdf?subformat=pdfa.
- Klafki, H.-W. et al. and Wiltfang, J. (2022) Title not specified. Fluids and Barriers of the CNS.
- Leuzy, A., et al. (2025). Considerations in the clinical use of amyloid PET and CSF biomarkers for Alzheimer’s disease. Alzheimer s & Dementia, 21(3). DOI: 10.1002/alz.14528. https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/alz.14528.
- Morgado, B., et al. (2024). Assessment of immunoprecipitation with subsequent immunoassays for the blood-based diagnosis of Alzheimer’s disease. European Archives of Psychiatry and Clinical Neuroscience, 275(8), pp.2215–2227. DOI: 10.1007/s00406-023-01751-2. https://link.springer.com/article/10.1007/s00406-023-01751-2.
- Shahpasand-Kroner, H. et al. and Wiltfang, J. (2018) Title not specified. Alzheimer’s Research & Therapy.
- Sims, J.R., et al. and TRAILBLAZER-ALZ 2 Investigators (2023). Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA, (online) 330(6). DOI: 10.1001/jama.2023.13239. https://jamanetwork.com/journals/jama/fullarticle/2807533.
- van Dyck, C.H., et al. (2022). Lecanemab in Early Alzheimer’s Disease. New England Journal of Medicine, (online) 388(1), pp.9–21. DOI: 10.1056/nejmoa2212948. https://www.nejm.org/doi/10.1056/NEJMoa2212948.
- Vogelgsang, J., et al. (2024). Plasma amyloid beta X‐42/X‐40 ratio and cognitive decline in suspected early and preclinical Alzheimer’s disease. Alzheimer’s & Dementia, (online) 20(8), pp.5132–5142. DOI: 10.1002/alz.13909. https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/alz.13909.
About IBL International GmbH, Part of Tecan Group
Specialty diagnostics to improve people’s lives and health
With decades of experience, Tecan has built a strong legacy of innovation in in vitro diagnostic testing for endocrinology, immunology and autoimmunity at IBL International, specializing in the development, manufacture and supply of immunoassays as well as LC-MS solutions. These products are designed and produced to the highest standards, providing diagnostics labs with reliable data and improved workflow efficiency to assess various health conditions from blood, urine, saliva and cerebrospinal fluid samples.
Tecan’s reagents portfolio includes a number of specialty diagnostic assays for endocrinology, immunology, neurotransmitters and autoimmunity in clinical diagnostics, along with key assays for the research segment, including BD-Tau and NF Light®*.
By combining Tecan’s proven automation capabilities and leadership in instrumentation with IBL International’s specialized immunoassay and LC-MS portfolio, Tecan offers complete solutions tailored to the needs of specialty diagnostics and research laboratories. These offerings streamline lab workflows by boosting productivity, increasing efficiency and meeting high regulatory standards.
This focus on compliance helped Tecan respond quickly to the EU’s In Vitro Diagnostic Regulation (IVDR), becoming one of the first companies to achieve product certification under the new rules.
Tecan continues to invest in innovation, advancing its portfolio to address emerging diagnostic needs in both clinical and research settings. Recent developments include specialized assays such as BD-Tau, sIL-2R, SCCA2 and Periostin ELISAs, supporting neurology and immunology applications, as well as LC-MS based assays for vitamins B1 and B6, A and E testing in endocrinology.
Disclaimer:
Products manufactured and distributed by IBL International. Availability and regulatory status may vary across regions depending on local country specific
registration. The combined use of the reagents, process script and instrument has to be validated individually on site by each laboratory.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.net, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.