The identification and validation of biomarkers for neurodegenerative diseases - particularly Alzheimer’s disease (AD) - is advancing rapidly. These biomarkers play a critical role in enabling early diagnosis, tracking disease progression, and assessing how patients respond to treatment.
Image Credit: Lightspring/Shutterstock.com
Traditionally, the AT(N) framework1 has guided the use of amyloid, tau, and neurodegeneration markers, primarily through imaging and cerebrospinal fluid (CSF) analysis. However, translating these markers to blood-based assays has proven challenging, in particular for total tau, which is confounded by peripheral sources and non-specific elevations in various neurological and systemic conditions.
This review explores the development and clinical utility of plasma brain-derived tau (BD-Tau), a novel biomarker designed to overcome these limitations, and explores its role in both chronic neurodegenerative and acute neurological conditions.
Tau and Alzheimer's Disease
Limitations of total tau and the need for CNS-specific biomarkers
Total tau has long been recognized as a marker of neurodegeneration in CSF, but its utility in blood is limited. The majority of blood total tau originates from peripheral tissues, not the CNS, and is elevated in a range of conditions, including Creutzfeldt-Jakob disease (CJD), head trauma, and anoxia, among others.
This lack of specificity has led to its removal from the revised AT(N) criteria for AD diagnosis2. The revised criteria highlight that total tau increases in many conditions unrelated to AD, making it unclear what total tau truly reflects in plasma.
The biological rationale and development of BD-Tau
Tau protein exists in multiple isoforms, with CNS tau characterized by low molecular weight forms and peripheral nervous system (PNS) tau by a larger isoform known as “big tau.”
Traditional total tau assays use antibodies that target regions shared by CNS and PNS tau, yielding a mixed signal in blood. To address this, the speaker’s team collaborated to develop a tau junction antibody (tau J), which specifically binds the junction between exon 4 and 5 – unique to CNS tau.
This antibody does not bind if the “big tau” insert is present, thus excluding peripheral tau from detection. The antibody was tested using ELISA and immunoprecipitation-mass spectrometry (IPMS), and an immunoassay was developed on the Simoa platform3.
Clinical validation in Alzheimer’s disease
Initial studies using neuropathologically confirmed cohorts demonstrated that plasma BD-Tau levels increase in the presence of AD pathology, in contrast to total tau, which remains unchanged across the AD continuum.
Memory clinic cohorts from Italy further confirmed that plasma BD-Tau is significantly elevated in AD compared to other neurological conditions, mirroring the specificity of CSF total tau. Unlike neurofilament light chain (NFL), which is elevated in various neurodegenerative diseases, BD-Tau shows greater specificity for AD3.
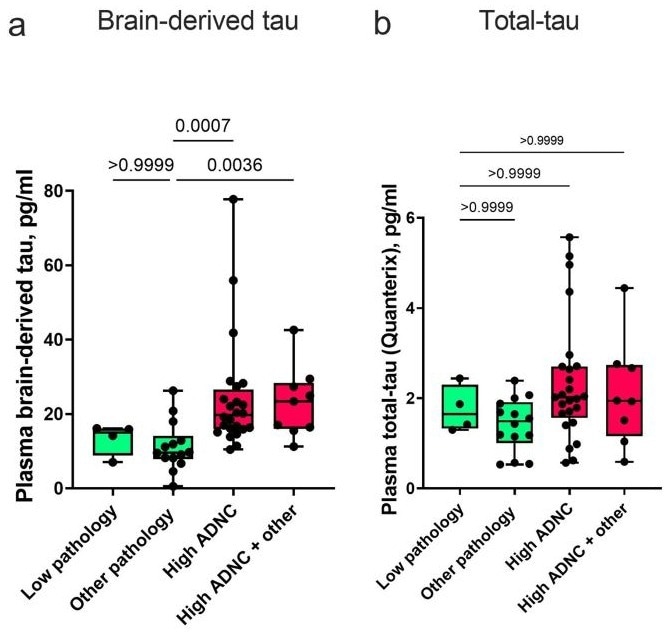
Image Credit: Gonzalez-Ortiz F, et al. Brain-derived tau: a novel blood-based biomarker for Alzheimer's disease-type neurodegeneration. Brain. 2023;146(3):1152-1165. doi:10.1093/brain/awac407
Further research in collaboration with Norwegian cohorts (dementia disease initiation cohort) revealed that plasma BD-Tau not only distinguishes AD from other conditions but also predicts longitudinal cognitive decline and changes in MRI meta-ROI signatures. Importantly, patients who are double positive for amyloid (phospho-tau 217 or 181) and BD-Tau in plasma exhibit the fastest rates of cognitive and structural decline, suggesting that BD-Tau adds prognostic value beyond amyloid markers alone4.
BD-Tau in acute neurological injury
The utility of BD-Tau extends beyond chronic neurodegeneration. In traumatic brain injury (TBI), plasma BD-Tau remains elevated for up to seven days post-injury, distinguishing patients with poor outcomes from those with better recovery. This sustained elevation contrasts with total tau and phospho-tau forms, which tend to normalize more quickly.
In acute ischemic stroke, BD-Tau measured at admission is the only marker capable of differentiating patients with good versus poor 90-day functional outcomes. The correlation between plasma BD-Tau and stroke lesion size is strong and independent of vascular territory or anatomical location, making it a sensitive marker for CNS injury5.
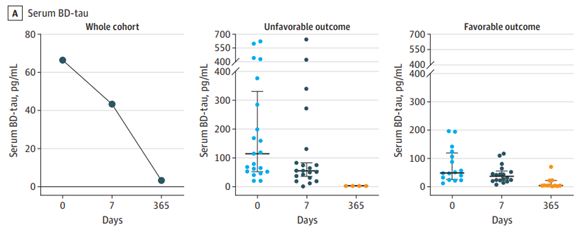
Image Credit: Gonzalez-Ortiz, F., et al. (2023). Association of Serum Brain-Derived Tau With Clinical Outcome and Longitudinal Change in Patients With Severe Traumatic Brain Injury. JAMA network open, 6(7), e2321554
Insights from rapidly progressive dementias
In rapidly progressive dementias such as CJD, plasma BD-Tau mirrors the dramatic increases seen in CSF total tau, reflecting the intensity of neurodegeneration. BD-Tau outperforms other markers, including total tau, NFL, and phospho-tau 217, as a predictor of survival in CJD patients.
The ratio of phospho-tau 217 to BD-Tau in plasma provides excellent diagnostic accuracy in distinguishing CJD from AD, highlighting the nuanced behavior of tau isoforms in different disease contexts6.
Broader applications and peripheral conditions
Preliminary data suggest that plasma BD-Tau may serve as an outcome biomarker in conditions with CNS involvement, such as Guillain-Barré syndrome, in which CNS involvement is associated with poorer outcomes.
BD-Tau has also shown potential value in neurosurgical and infectious disease settings, indicating its broader applicability as a marker of CNS injury7,8,9.
Implications for biomarker research
The findings challenge the notion that phospho-tau markers are exclusively AD or amyloid-dependent. Elevations in phospho-tau 217 and other forms have been observed in CJD and after TBI, suggesting that these markers are amyloid-associated but not strictly amyloid-dependent.
Such findings highlight the necessity for more specific assays that can distinguish CNS-derived tau from peripheral sources and accurately reflect neurodegenerative processes.
Conclusion
Plasma BD-Tau, enabled by the use of tau junction antibody, represents a significant advancement in neurodegenerative and acute neurological biomarker research. Its CNS specificity allows for accurate detection of neurodegeneration in AD, prediction of cognitive decline, and monitoring of structural brain changes.
In acute neurological injuries such as TBI and stroke, BD-Tau provides prognostic information and correlates with lesion size, independent of anatomical location. In rapidly progressive dementias, BD-Tau serves as a robust survival predictor and aids in differential diagnosis.
The future of tau biomarker research lies in combining the tau junction antibody with phospho-tau markers to further enhance specificity and diagnostic utility. This approach may help overcome peripheral contamination and improve the accuracy of blood-based assays.
Plasma BD-Tau holds promise as both a diagnostic and prognostic biomarker, complementing existing markers and informing clinical decision-making in a wide range of neurological conditions.
In summary, plasma BD-Tau is poised to become a valuable tool for identifying patients at risk of rapid cognitive decline in AD, guiding clinical trial enrollment, and providing prognostic insights in acute neurological disorders. Ongoing research will clarify its role in predicting therapy response and its utility in other CNS and peripheral conditions, paving the way for more personalized and effective approaches to neurological care.
References
- Jack, C.R., et al. (2018). NIA-AA research framework: Toward a biological definition of alzheimer’s disease. Alzheimer’s & Dementia : the Journal of the Alzheimer’s Association, (online) 14(4), pp.535–562. DOI: 10.1016/j.jalz.2018.02.018. https://alz-journals.onlinelibrary.wiley.com/doi/10.1016/j.jalz.2018.02.018.
- Jack, C.R., et al. (2024). Revised criteria for the diagnosis and staging of Alzheimer’s disease. Nature Medicine. DOI: 10.1038/s41591-024-02988-7. https://www.nature.com/articles/s41591-024-02988-7.
- Gonzalez-Ortiz, F., et al. (2022). Brain-derived tau: a novel blood-based biomarker for Alzheimer’s disease-type neurodegeneration. Brain, 146(3). DOI: 10.1093/brain/awac407. https://academic.oup.com/brain/article/146/3/1152/6960988?login=false.
- Gonzalez-Ortiz, F., et al. (2024). Plasma brain-derived tau is an amyloid-associated neurodegeneration biomarker in Alzheimer’s disease. Nature communications, 15(1). DOI: 10.1038/s41467-024-47286-5. https://www.nature.com/articles/s41467-024-47286-5.
- Gonzalez-Ortiz, F., et al. (2023). Association of Serum Brain-Derived Tau With Clinical Outcome and Longitudinal Change in Patients With Severe Traumatic Brain Injury. JAMA Network Open, (online) 6(7), pp.e2321554–e2321554. doi:https://doi.org/10.1001/jamanetworkopen.2023.21554.
- Bentivenga, G.M., et al. (2024). Clinical value of novel blood‐based tau biomarkers in Creutzfeldt–Jakob disease. Alzheimer’s & Dementia, 21(2). DOI: 10.1002/alz.14422. https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/alz.14422. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2806834.
- Hafsteinsdóttir, B., et al. (2025). Brain‐Derived Tau as an Outcome Marker in Guillain‐Barré Syndrome: A Retrospective Cohort Study. European Journal of Neurology, 32(4). DOI: 10.1111/ene.70155. https://onlinelibrary.wiley.com/doi/10.1111/ene.70155?utm_source=researchgate.net&utm_medium=article.
- Ørbæk, M., et al. (2025). Plasma levels of the neuron damage markers brain-derived tau and glial fibrillary acidic protein in Lyme neuroborreliosis: A longitudinal study. Ticks and Tick-borne Diseases, 16(3), p.102459. DOI: 10.1016/j.ttbdis.2025.102459. https://www.sciencedirect.com/science/article/pii/S1877959X25000238?via%3Dihub.
- Svedung Wettervik, T., et al. (2025). Blood biomarkers for brain injury in chronic subdural hematomas: postoperative dynamics and relation to long-term outcome. Journal of Neurosurgery, pp.1–11. DOI: 10.3171/2025.1.jns242942. https://pubmed.ncbi.nlm.nih.gov/40250046/.
About IBL International GmbH, Part of Tecan Group
Specialty diagnostics to improve people’s lives and health
With decades of experience, Tecan has built a strong legacy of innovation in in vitro diagnostic testing for endocrinology, immunology and autoimmunity at IBL International, specializing in the development, manufacture and supply of immunoassays as well as LC-MS solutions. These products are designed and produced to the highest standards, providing diagnostics labs with reliable data and improved workflow efficiency to assess various health conditions from blood, urine, saliva and cerebrospinal fluid samples.
Tecan’s reagents portfolio includes a number of specialty diagnostic assays for endocrinology, immunology, neurotransmitters and autoimmunity in clinical diagnostics, along with key assays for the research segment, including BD-Tau and NF Light®*.
By combining Tecan’s proven automation capabilities and leadership in instrumentation with IBL International’s specialized immunoassay and LC-MS portfolio, Tecan offers complete solutions tailored to the needs of specialty diagnostics and research laboratories. These offerings streamline lab workflows by boosting productivity, increasing efficiency and meeting high regulatory standards.
This focus on compliance helped Tecan respond quickly to the EU’s In Vitro Diagnostic Regulation (IVDR), becoming one of the first companies to achieve product certification under the new rules.
Tecan continues to invest in innovation, advancing its portfolio to address emerging diagnostic needs in both clinical and research settings. Recent developments include specialized assays such as BD-Tau, sIL-2R, SCCA2 and Periostin ELISAs, supporting neurology and immunology applications, as well as LC-MS based assays for vitamins B1 and B6, A and E testing in endocrinology.
Disclaimer:
Products manufactured and distributed by IBL International. Availability and regulatory status may vary across regions depending on local country specific registration. The combined use of the reagents, process script and instrument has to be validated individually on site by each laboratory.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.net, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.