As the COVID-19 pandemic continues to claim lives throughout the world, the evidence is mounting that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) affects the respiratory tract as well as the central nervous system. Now, a new study by German researchers published on the preprint server bioRxiv* in June 2020 describes various neurological manifestations of COVID-19, as well as the underlying pathophysiology.
While COVID-19 continues to be primarily a respiratory disorder, severe disease is accompanied by many other events, including thromboembolism in the central nervous system. The occurrence of neurological symptoms such as anosmia, ageusia, and headache in most patients indicates that the virus penetrates the brain.
This is supported by the occurrence of acute stroke-related disorders and reduced or altered consciousness in some patients with COVID-19. Moreover, many recent papers show that viral RNA is detectable in the brain and cerebrospinal fluid (CSF).
Mapping Virus-Brain Correlations
The current study aims to achieve an accurate exploration of the oropharyngeal regions and brains of 32 patients with fatal COVID-19, tracing the symptoms to the affected regions. This autopsy-based study is aimed at identifying the port of entry of the virus into the brain, and its distribution.
Earlier research has shown that coronaviruses related to SARS-CoV-2 have the potential to invade the brain. Mouse studies engineered to express the human angiotensin-converting enzyme 2 (ACE2) receptor show that the virus can enter the brain when inoculated intranasally. On the other hand, the olfactory cells that express these molecules either at baseline or when inflamed or infected are unidentified as yet. In this context, it is noteworthy that the molecule CD147 expressed on various cells in the brain, and belonging to the immunoglobulin superfamily, is another port for SARS-CoV-2 invasion into those cells.
The researchers examined the microenvironment of the mucous membranes and the neuronal tissue, as the site of viral entry and replication. Next, they mapped the whole of the olfactory nervous tracts region by region, as well as certain other specific regions, in 32 brains from individuals who had lethal COVID-19 infections.
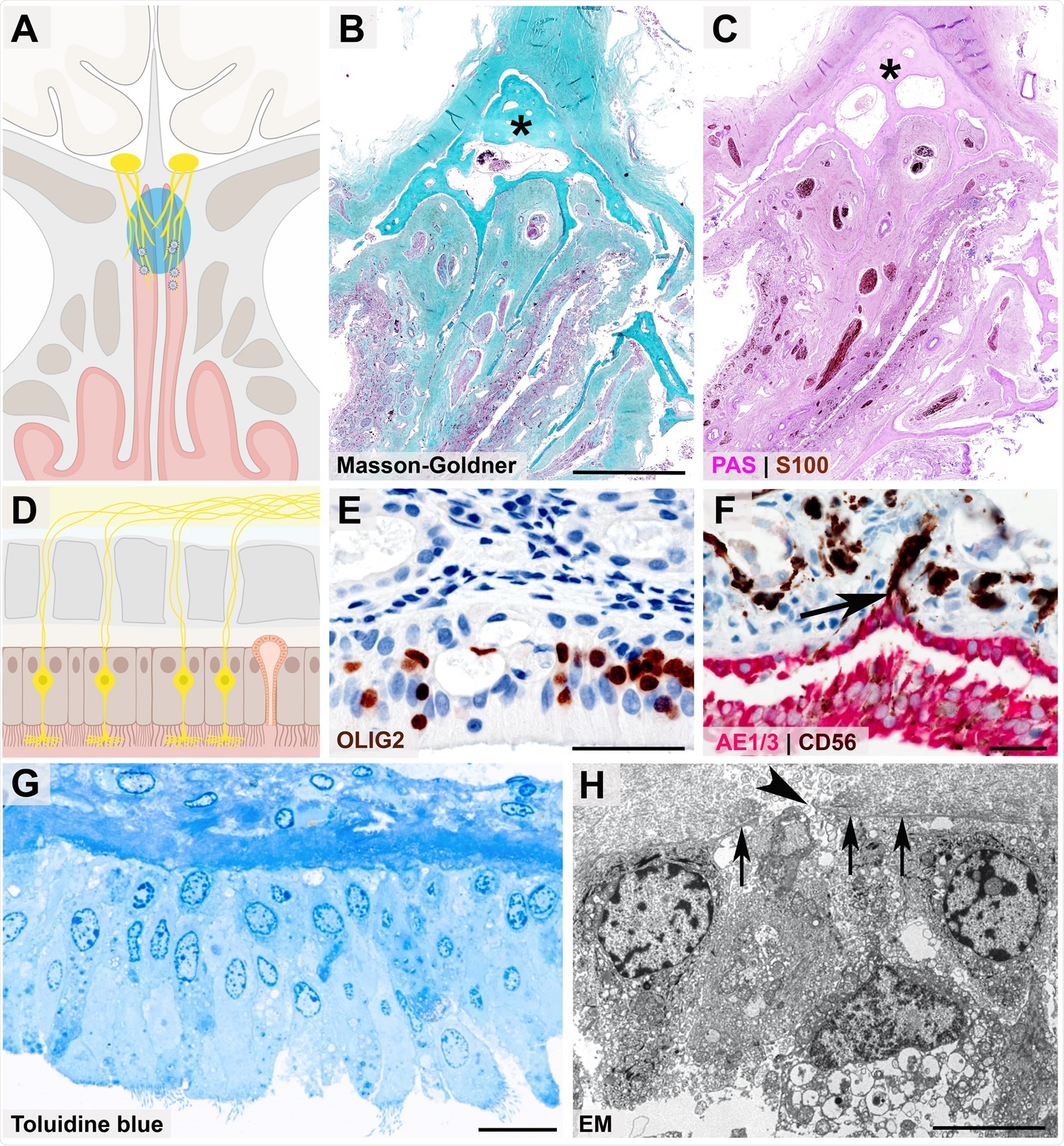
Close anatomical proximity of nervous and epithelial tissues in the olfactory mucosa Cartoon (A) and histopathological coronal cross-sections (B - C) depicting the paranasal sinus region with the osseous cribriform plate (turquoise color and asterisk in B, pink color and asterisk in C) and the close anatomical proximity of the olfactory mucosa (green in B, purple in C) and nervous tissue characterized by nerve fibers immunoreactive for S100 protein (C, brown color). Cartoon (D) resembling the olfactory mucosa, which is composed of pseudostratified ciliated columnar epithelium, basement membrane, and lamina propria, also containing mucus-secreting Bowman glands and bipolar olfactory receptor neurons (ORNs), which coalesce the epithelial layer. 368 Immunohistochemical staining of the olfactory mucosa (E, F) showing nuclear expression of OLIG2 specifying late neuronal progenitor and newly formed neurons (E, brown color)31 369, which are closely intermingled with epithelial cells (F, immunoreactivity for the pancytokeratin marker AE1/3, red color). The basement membrane underneath the columnar AE1/3-positive epithelium (F, red color) is discontinued due to CD56-positive (F, brown color) axonal projections of ORNs (F, arrow). The ORN 3dendrite carries multiple cilia and protrudes into the nasal cavity (G, semithin section, toluidine blue staining), while the axon (H, arrowhead) crosses the olfactory mucosa basement membrane (H, 3arrows) as a precondition for ORN projection into the glomeruli of the olfactory bulb, which is readily 3visible at the ultrastructural level). Scale bars: B: 3.5 cm; E, F: 50 µm; G: 20 µm; H: 5 µm.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
COVID-19 and The Olfactory Mucosa
Of the 32 autopsy cases, of which 29 were RT-PCR positive for SARS-CoV-2 and the remaining three diagnosed on the basis of their signs and symptoms, there were four patients, or 13%, with acute cerebral infarction caused by ischemia, resulting from microscopic clots or emboli within the brain. Similar micro-clots were also seen in the olfactory mucosa.
The viral load was most significant in the olfactory mucosal cells just below the cribriform plate, followed by the cornea, conjunctiva, and the mucous membrane of the mouth. This indicates that while the respiratory route may be primary for the entry of the virus into the CNS, the eyes and the mouth also offer alternative pathways. In about 10%, the virus was also detected within the cerebellum.
The presence of subgenomic viral RNA was taken to denote that viral replication was taking place. This was observed only in 4 of the 13 PCR-positive samples of olfactory mucosa and in 2 of 6 positive uvulae.
Viral markers in the CNS was more likely in patients with a shorter duration of disease. The fact that COVID-19 patients have anosmia and ageusia, coupled with the fact that the neurons, nerve fibers, and mucosa within the oropharynx and mucopharynx are so near each other, could indicate that the mucosa is a gateway for the viral invasion of the CNS.
The olfactory neurons project into the olfactory mucosa in the nose, while on the other side, they merge into long thin processes called fila that penetrate the cribriform plate to reach the olfactory bulb. This also gives them access to the CNS.
Immunohistochemistry shows that the virus specifically infects these cells, while activated macrophages initiate the immune response. This acts as the damage-associated molecular pattern (DAMP) that leads to TLR4-Myd88 signaling.
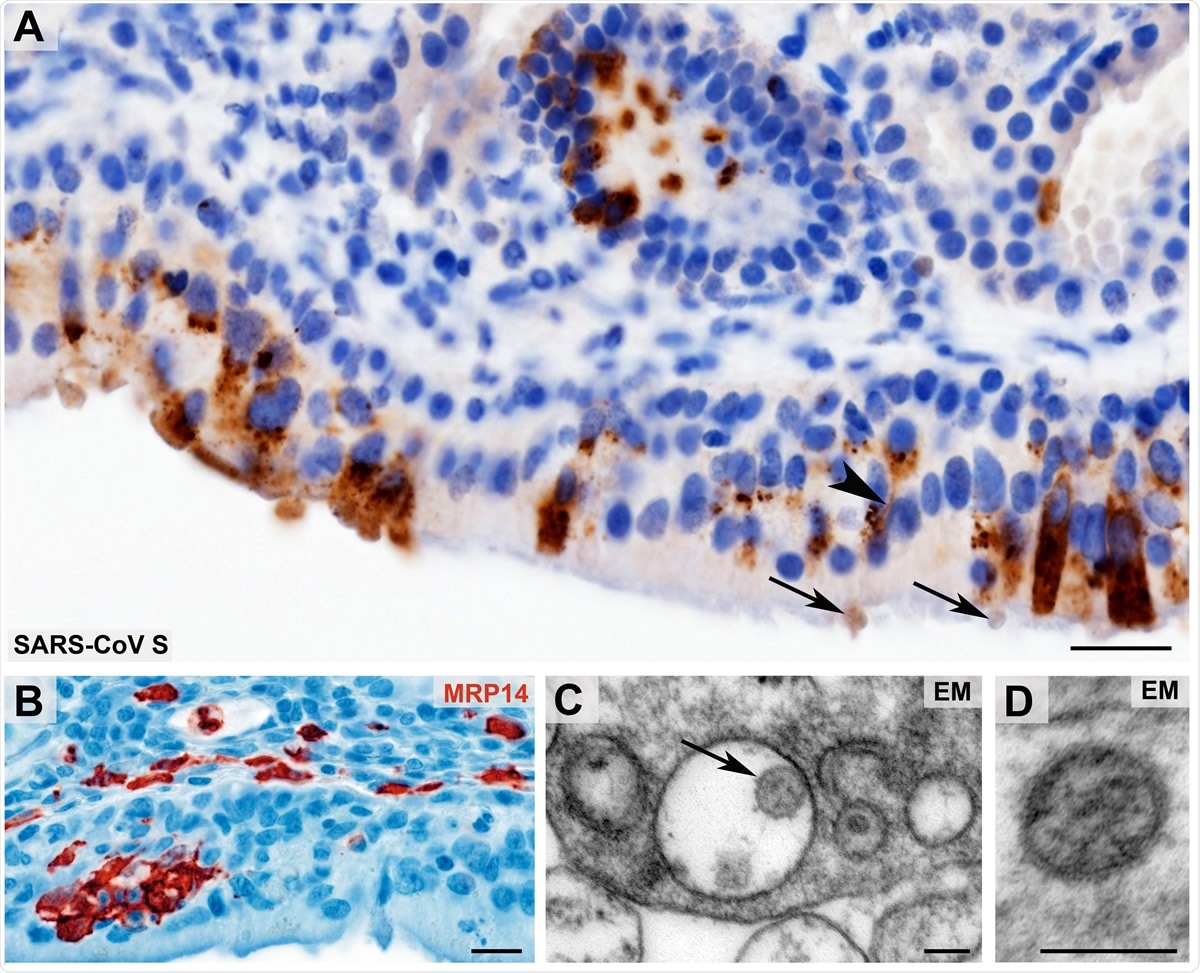
Morphological evidence of SARS-CoV presence and first innate immune cell response within the olfactory mucosa Coronavirus antigen (A, SARS-CoV Spike Protein (SARS-CoV S), brown color) exhibits a cytoplasmic staining with perinuclear accentuation of infected mucosal (epithelial) cells and identifies SARS-CoV383 positive dendrites (arrowhead) and vesicles at the dendrite tips (arrows) of the olfactory receptor neurons. Small clusters of infiltrating, early activated macrophages and granulocytes (MRP14, red color) in the olfactory epithelium upon SARS-CoV-2 infection (B). Ultrastructural images of two different examples of Coronavirus-like particles in the olfactory mucosa (C - D; arrow in C) fulfilling the criteria of size, shape, structural features (membrane, surface structures, electron dense material within the particle, resembling ribonucleoprotein) and localization (C, cytoplasmic localization within a membrane compartment, sometimes with typical attachment on the inner membrane surface as 3shown in this example; D, extracellular). Scale bars: A, B: 20 µm; C, D: 100 nm
Implications and Hypotheses
The study thus reveals for the first time that the virus invades the CNS at the neuro-mucosal interface through the olfactory nerve receptor fibers, tracking along the olfactory tract and thus explaining the occurrence of anosmia and ageusia. The presence of tiny clots and brain infarcts in this territory in 13% of the brain samples seems to corroborate other reports of thromboembolism in the CNS of affected patients.
While the current data may seem to favor axonal transport as the mode of neuroinvasion by SARS-CoV-2, other routes are possible, such as across the synapses, via the endothelium, or within leukocytes migrating across the blood-brain barrier.
Finally, the olfactory mucosa seems to be an ongoing site of viral persistence and replication, judging from the persistently high levels of the virus in this tissue for up to 53 days from initial symptoms. The authors also suggest that possibly the virus present in the vital centers of the brain may cause worsening of pre-existing respiratory or cardiac issues via a central mechanism. It may even spread more extensively within the CNS, though evidence for this is lacking at present.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Meinhardt, J., et al. (2020). Olfactory Transmucosal SARS-Cov-2 Invasion as Port Of Central Nervous System Entry In COVID-19 Patients. bioRxiv preprint. doi: https://doi.org/10.1101/2020.06.04.135012. https://www.biorxiv.org/content/10.1101/2020.06.04.135012v1
- Peer reviewed and published scientific report.
Meinhardt, Jenny, Josefine Radke, Carsten Dittmayer, Jonas Franz, Carolina Thomas, Ronja Mothes, Michael Laue, et al. 2020. “Olfactory Transmucosal SARS-CoV-2 Invasion as a Port of Central Nervous System Entry in Individuals with COVID-19.” Nature Neuroscience 24 (2): 1–8. https://doi.org/10.1038/s41593-020-00758-5. https://www.nature.com/articles/s41593-020-00758-5.