Offer the clinical confidence that patients deserve using the DCA Vantage Analyzer from Siemens Healthineers. It helps users track glycemic control and detect early kidney disease in environments ranging from the physician's office to remote, point-of-care coordinated sites in multisite and hospital practices.
Fulfill lab-quality testing standards with an analyzer that expedites and eases diabetes tests and delivers precise, clinically relevant outcomes that enhance patient compliance, decision-making, and outcomes.1,2,3,4
- Manage diabetes patients in a more effective manner
- Streamline management of diabetes testing in decentralized settings
- Workflow in the office or clinic can be enhanced
- One of just two HbA1c analyzers that fulfill NGSP performance criteria1
- Utilized by three out of four physicians who conduct HbA1c testing in their office5
DCA vantage analyzer
Actionable results in seven minutes or less
Users can reduce the need for follow-up visits with a complete spectrum of outcomes. HbA1c (NGSP certified and IFCC certified/CLIA waived), Albumin, Estimated Average, Creatinine, and Albumin-to-Creatinine Ratio outcomes.

Image Credit: Siemens Medical Solutions USA, Inc
Easy to use in the office or clinic
There is no need for sample or reagent preparation, and the system offers adaptable data management and reporting options.

Image Credit: Siemens Medical Solutions USA, Inc
Robust technology delivers proven performance
The analyzer leverages robust technology and offers the proven performance of the DCA HbA1c and DCA Microalbumin/Creatinine tests stressed in over 140 clinical articles.
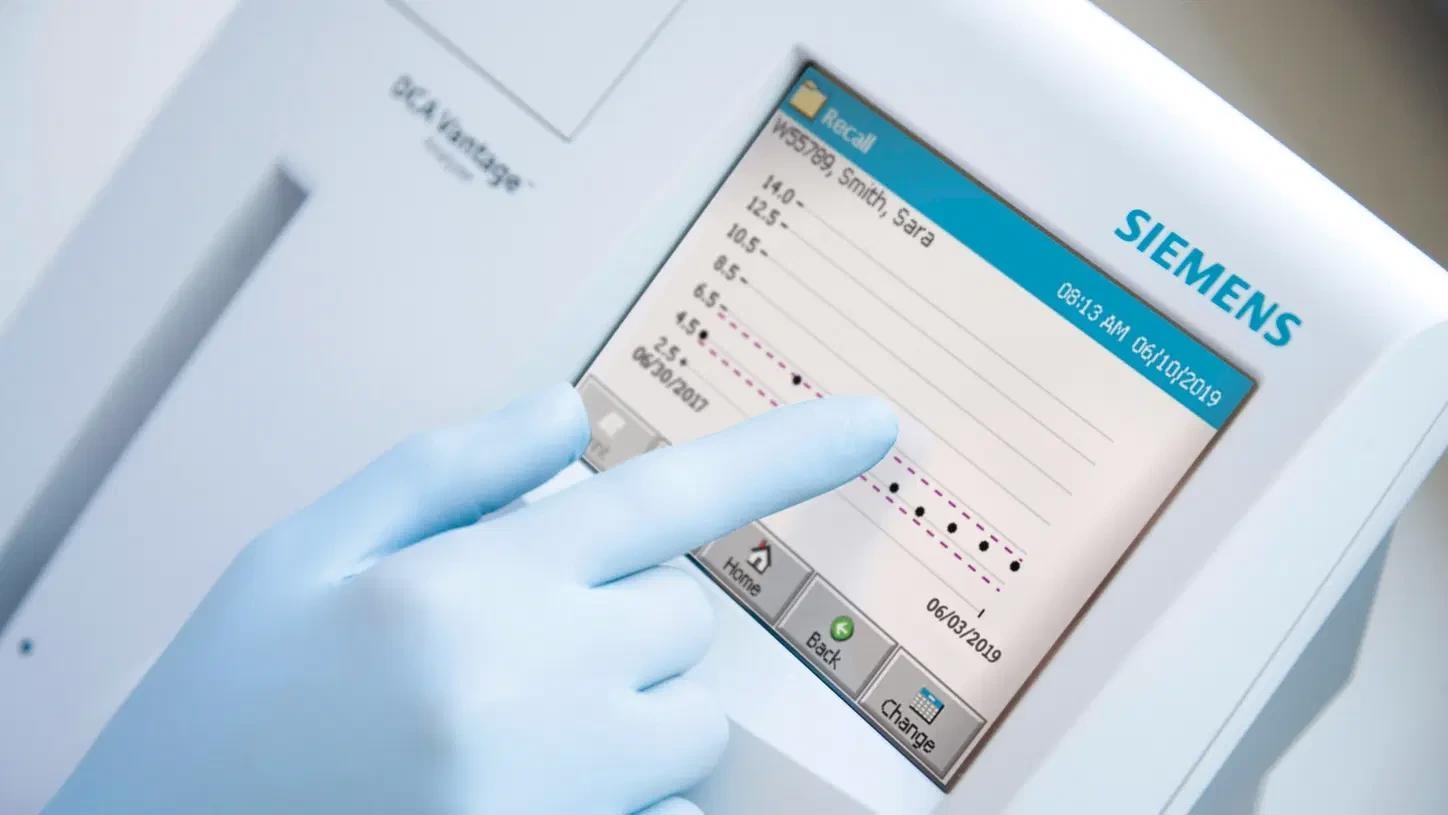
Image Credit: Siemens Medical Solutions USA, Inc
Manage diabetes testing in decentralized settings
Robust functionality allows POC coordinators to regulate diabetes testing in decentralized settings better. While supported by Siemens Healthineers POCcelerator Data Management System, coordinators could oversee and troubleshoot several connected analyzers in real-time.

Image Credit: Siemens Medical Solutions USA, Inc
Features and benefits
Users can track glycemic control and diabetes complications with the help of an analyzer developed to simplify consultations. Rapid and actionable test outcomes allow users to identify the effectiveness of a treatment plan, confidently make therapeutic adjustments, and be more evident if patients comply with users’ suggestions.
Monitor glycemic control
- HbA1c from a small (1 µL) whole blood sample can be collected in just six minutes
- Adaptable reporting of HbA1c% (JDS, NGSP, and Mono-S units) and IFCC (mmol/mol)
- HbA1c patient trending graphs could be viewed or printed
- Reporting of HbA1c outcomes as Estimated Average Glucose values in the same units (mg/dL) that patients’ home glucose meters tend to display
Detect early kidney disease
- Creatinine, Albumin, and Albumin-to-Creatinine (A:C) ratio from a urine specimen in just seven minutes to report a quantitative protein status with the help of automatic creatinine adjustment
- Onboard GFR calculator indexes kidney function
Improve workflow in the office or clinic
- Self-contained cartridges enable simple and walk away operations following sample loading
- No sample or reagent preparation needed
- Bar-code scanner available for safer and quicker patient or operator ID entry
- Upload results to a PC via a USB flash drive automatically to decrease manual logging and save time
- Users can review outcomes on-screen or produce a hard copy report to reduce transcription errors in the office
- Comfortable local storage of up to 4,000 onboard records with strong sorting abilities
- Reduce maintenance needs with automatic reminders to alert users when maintenance is due
Simplify management of diabetes testing in decentralized settings
- The availability of customizable security access modes assists up to 1,000 operators, protects patient information, and avoids operation by unauthorized users
- Automatically upload outcomes and QC information to LIS/HIS to decrease manual logging and save time
- POCT1-A2 communication protocol eases data transfer for simple connectivity and quick, two-way communication to LIS/HIS, POCcelerator System, or other third-party POC data management systems
- Users can take testing supervision to the next level by remotely managing several analyzers and operators to improve compliance and risk management.
POC coordinators could enforce QC protocols, standardize test procedures, control access privileges, specify operator recertification needs, and more. Customized alerts, reports, and audit trails guarantee peace of mind and facilitate inspections and accreditation.
Assays
Formulas for calculated results
- % HbA1c = (HbA1c/Total Hemoglobin) x 100
- eAG* mg/dL = (28.7 × HbA1C) – 46.7
- eAG* mmol/L = (1.59 × HbA1C) – 2.59
- GFR = 186 x (plasma creatinine mg/dL)-1.154 x (patient age years) -0.203 × (0.742 if female patient) × (1.210 if African American patient)
Formulas for dual reporting from IFCC to % HbA1c
- NGSP = (0.09148 x IFCC) + 2.152
- JDS = (0.09274 x IFCC) +1.724
- Mono-S = (0.09890 x IFCC) + 0.884
Formulas for dual reporting from % HbA1c to IFCC mmol/mol
- IFCC = (10.93 x NGSP) – 23.50
- IFCC = (10.78 x JDS) – 18.59
- IFCC = (10.11 x Mono-S) – 8.94
Technical specifications
Source: Siemens Medical Solutions USA, Inc
| Overview |
| System Description |
Point-of-care immunoassay analyzer |
| Quantitative Tests |
Hemoglobin A1c (whole blood): Range: 2.5% to 14% (4mmol/mol to 130 mmol/mol) Microalbumin/Creatinine (urine): Single test reports all three results for: Albumin: 5 to 300 mg/L; Creatinine: 15 to 500 mg/dL (1.3 to 44.2 mmol/L); Albumin-to Creatinine Ratio: 1 to 2000 mg/g (0.11 to 226 mg/mmol) |
| Test Format |
Self-contained immunoassay cartridges |
| Test Measurement |
Automatic, optional transmission |
| Test Method |
HbA1c: monoclonal antibody agglutination reaction
Albumin: polyclonal goat anti-human albumin antiserum
Creatinine: Benedict Behre chemical reaction |
| Time to Test Results |
HbA1c - 6 minutes
A:C Ratio - 7 minutes |
| Test Handling |
| Sample Volume |
HbA1c - 1 µL whole blood
Microalbumin/Creatinine - 40 µL urine |
| Sample Preparation |
No pretreatment; no pipetting required |
| Sample ID/Operator ID Entry |
Optional; via touch screen or bar code reader |
| Calibration |
| Calibration |
Lot-specific calibration card provides automatic calibration with every cartridge
Traceable to International Federation of Clinical Chemistry (IFCC) reference materials and test methods for measurement of HbA1c |
| Onboard Computer |
| Storage Capacity/Memory |
4000 patient and/or control records
Up to 1,000 operator IDs |
| Display |
Color touch screen with 1/4 VGA resolution |
| Data Export |
Via USB flash drive to PC or direct to LIS/HIS or data manager, if interfaced |
| Quality Control/Compliance |
| Flexible QC Scheduling |
None, Automatic Reminders or Required |
| QC Testing |
Optional lockout if schedule not followed or QC fails |
| User/Operator Access |
Restricted, if desired, to protect patient and QC data and prevent unauthorized use |
| Matching Lab Results/ Reference Method |
Adjustable correlation to reference methods |
| Reference Ranges |
User-definable reference ranges available for HbA1c |
| Computer/Peripheral Interfaces |
| Serial Port |
RS232, ASTM |
| Ethernet Connection |
ASTM or POCT1-A2 |
| Bi-Directional Capabilities |
ASTM: Remote computer can be set up to lock out patient tests
POCT1-A2: Remote computer can be set up to lock out patient tests, and send operator list to analyzer |
| USB-Port |
Standard USB 2.0 |
| External Bar Code Reader (optional) |
Serial (9 pin) |
| Onboard Printer |
54 mm (2 in) width, thermal/label stock |
| External Printer |
Supports standard PCL printer interface via USB port |
| General |
| Dimensions |
9.0 (h) x 11.5 (w) x 10.5 (d) inches 25.4 (h) x 28.7 (w) x 27.7 (d) cm |
| Weight |
3.88 kg (9.0 lb) |
| Power Requirements |
100 to 240 VAC; 50/60 Hz |
| Line Leakage Current |
<0.3 mA in normal condition<0.5 mA in single fault condition |
| Maximum Power Input |
70 VA; 30 watts |
| Ambient Operating Temperature |
18 °C to 30 °C (64 °F to 86 °F) (Albumin)
15 °C to 32 °C (61 °F to 88 °F) (HbA1c) |
| Operating Temperature |
5 °C to 40 °C (41 °F to 104 °F);
15% to 90% relative humidity |
| Safety |
TUV SUD with CB Scheme, CSA-C22.2,
EN60601, IEC 60601, UL60601 |
| EMC Emissions/Immunity |
FCC 47: Part 15 (Class B),
EN60601-1-2 (Class B) |