Transmembrane proteins (TPs) are proteins that traverse both the intracellular and extracellular environments and are embedded in the cell membrane. The transmembrane area, which interacts directly with the phospholipid bilayer, is a critical link between two environments, allowing for the transit of ions and molecules as well as conveying activation and response reactions to external stimuli.
These processes govern cell metabolism, cellular activity and cellular destiny by propagating intracellularly through downstream signal pathways. TPs could be characterized structurally as beta-barrels or alpha-helical, and topologically as single-pass or multi-pass molecules, depending on how the N and C termini are oriented.
The aberrant actions of these TPs have been linked to a range of human illnesses, and their position in a variety of signaling pathways makes them excellent therapeutic targets.
A full-length and physiologically relevant version of these proteins must be identified to create efficient therapeutics targeting these TPs.
Multi-pass TPs are notably hard to isolate due to their complicated architectures with numerous hydrophobic transmembrane sections and low expression levels in host cells, yet they have emerged as a popular disease target.
Rituximab detects CD20 not only at its primary epitope in extracellular loop 2 (ECL2) but also at its secondary epitope in extracellular loop 1 (ECL1). Similarly, Rituximab’s CDC activity is affected by this secondary epitope. To properly evaluate antibody activity and mode of action, full-length TPs with native folding are required.
Multiple technological platforms have been specifically developed by ACROBiosystems to solve the structural complexity of multi-pass TPs and to fulfill the demands of various applications.
To enhance drug development and mechanism research, ACRO has created a large variety of full-length multi-pass TPs with stable structure and high activity, such as CD20, Claudin18.2, GPRC5D, CD133, CCR5 and CCR8.
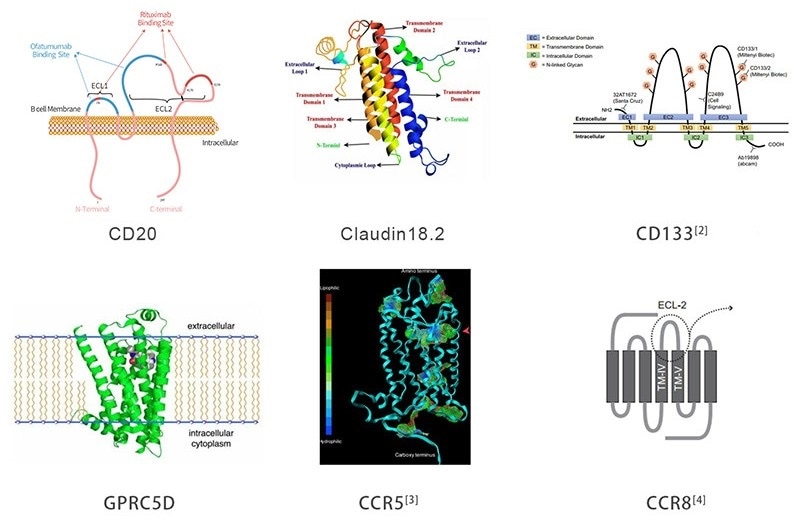
Figure 1. Multi-pass transmembrane target proteins. Image Credit: ACROBiosystems
Technology platforms
VLP platform
Principle
ACROBiosystems' VLP (Virus Like Particles) technological platform depends on the HEK293 expression system to express TPs on the host cell surface. The viral envelop or capsid protein subsequently converts these cell surfaces into soluble lipid bilayer particles containing highly concentrated proteins, which may be used for antibody immunization and screening.
The membrane protein-VLP complex induces and screens functional antibodies that identify the target’s normal configuration by displaying appropriately folded multi-pass TPs in its cell membrane. In addition to the range of VLP-based multi-pass TPs, ACRO also offers customized services.
Advantages
- Immunogenicity is higher
- Higher abundance than that of overexpressing cells
- Immunization, ELISA, SPR, BLI, cell experiment and CAR detection are all compatible
- Full ECD epitopes in full-length TPs
- Due to their 100–200 nm size, they may be employed as the ideal targets for dendritic cells for phage display in vivo.
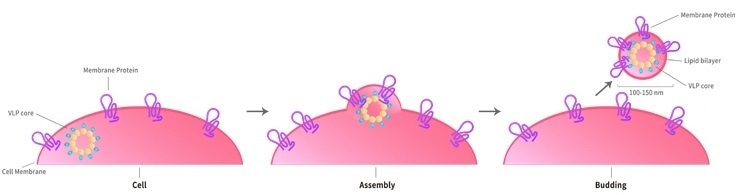
Image Credit: ACROBiosystems
Detergent micelle platform
Principle
As the transmembrane portion of multi-pass TPs is known to be highly hydrophobic, it is challenging to keep it in the right conformation once it has been removed from the cell membrane in standard buffers. Detergents can be added to resolve this problem.
For challenging therapeutic target TPs, ACROBiosystems has developed a full platform for insect cell and mammalian cell expression, purification and stabilization. To enhance solubility and assure the natural fold of this protein in vitro, ACRO uses detergent screening, including DDM/CHS (Cat. No. DC-11).
Advantages
- Can be precisely measured
- Immunization, ELISA, SPR and BL are all compatible
- TPs with complete conformation
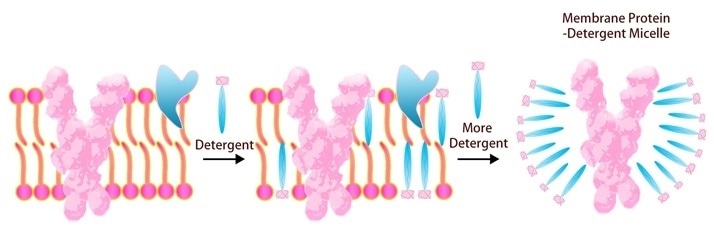
Image Credit: ACROBiosystems
Nanodisc platform
Principle
Membrane scaffold proteins (MSPs) and phospholipid molecules make up the so-called “Nanodisc,” a synthetic phospholipid bilayer membrane structure. After the detergent is removed, TP may be incorporated into the structure of Nanodisc to maintain its natural folding, biological activity and increased hydrophilicity for numerous applications.
In CAR expression assays, for example, the detergent-free formulation of Nanodisc-based TP is acceptable. The Nanodisc technology was licensed from the University of Illinois, and ACROBiosystems has been continuously optimizing and improving the assembly process to make it appropriate for commercial scale-up manufacturing.
ACRO’s efforts have provided the biopharma industry with a long-term steady supply of Nanodisc-based TP products.
Advantages
- Immunization/ELISA/SPR/BLI/cell experiment/CAR detection compatible
- Detergent-free high hydrophilicity
- Patent holder with a valid license
- Full-length TPs reside in a natural membrane environment, which allows them to retain high biological activity.
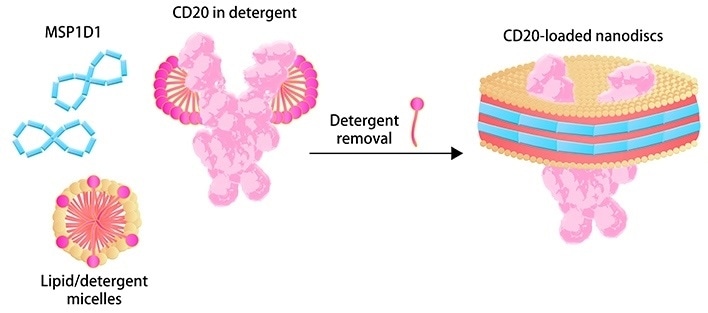
Image Credit: ACROBiosystems
Product list
VLP
Table 1. Source: ACROBiosystems
| Molecule |
Cat. No. |
Product Description |
Application |
| Claudin18.2 |
CL2-H5547 |
Human Claudin-18.2 Full Length Protein-VLP (HEK293) |
Immunization
ELISA
SPR
BLI
Cell Experiment
CAR Detection |
| Claudin18.2 |
CL2-H52P7 |
Human Claudin-18.2 Full Length Protein-VLP (HEK293, stable cell pool) |
| Claudin18.2 |
CL2-HF218 |
Fluorescent Human Claudin-18.2 Full Length Protein-VLP (HEK293) |
| GPRC5D |
GPD-H52P5 |
Human GPRC5D Full Length Protein-VLP (HEK293) |
Click here to learn about many more VLP.
Detergent micelle
Table 2. Source: ACROBiosystems
| Molecule |
Cat. No. |
Product Description |
Application |
| CD20 |
CD0-H52H3 |
Human CD20 / MS4A1 Full Length Protein, His Tag (HEK293) (SPR verified) |
Immunization
ELISA
SPR
BLI |
| CD20 |
CD0-H82E5 |
Biotinylated Human CD20 / MS4A1 Full Length Protein, His,Avitag™ (HEK293) |
| CD20 |
CD0-C52H8 |
Cynomolgus CD20 / MS4A1 Full Length Protein, His Tag |
| Claudin18.2 |
CL2-H5546 |
Human Claudin-18.2 Protein, His Tag (active membrane protein) |
Click here to learn about many more detergent micelles.
Nanodisc
Table 3. Source: ACROBiosystems
| Molecule |
Cat. No. |
Product Description |
Application |
| CD20 |
CD0-H52H1 |
Human CD20 / MS4A1 Full Length Protein, His Tag (Nanodisc) (HEK293) |
Immunization
ELISA
SPR
BLI
Cell Experiment
CAR Detection |
| CD20 |
CD0-H82E3 |
Biotinylated Human CD20 / MS4A1 Full Length Protein, His,Avitag™ (Nanodisc) (HEK293) |
| CD133 |
CD3-H52H1 |
Human CD133 Full Length Protein, His Tag (Nanodisc) |
| CD133 |
CD3-H82E6 |
Biotinylated Human CD133 Protein, His Tag (Nanodisc) |
Click here to learn about many more nanodiscs.
Case display
Claudin18.2-VLP
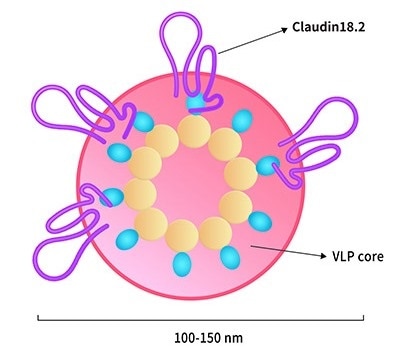
Claudin18.2-VLP. Image Credit: ACROBiosystems
Correct assembly validated by SEM
Under an electron microscope, full-length Claudin18.2-VLP (Cat. No.CL2-H5547) was examined to confirm that it was assembled appropriately.
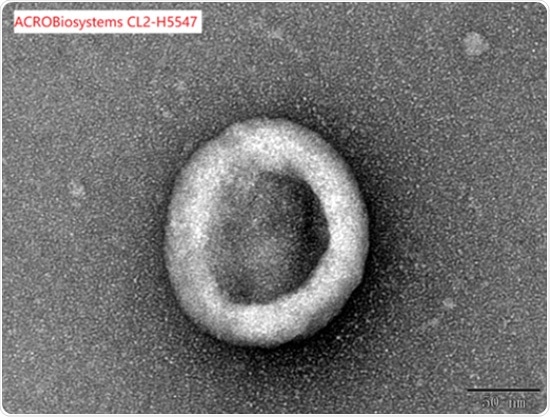
Image Credit: ACROBiosystems
Good bioactivity validated by ELISA
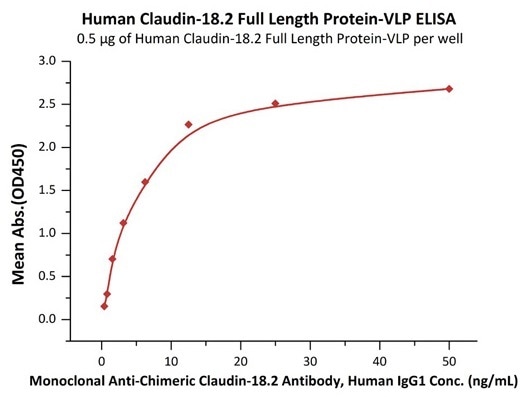
Immobilized Human Claudin-18.2 Full Length Protein-VLP (Cat. No. CL2-H5547) at 5 μg/mL (100 μL/well) can bind Monoclonal Anti-Chimeric Claudin-18.2 Antibody, Human IgG1 with a linear range of 0.8–3 ng/mL (QC tested). Image Credit: ACROBiosystems
High affinity validated by SPR
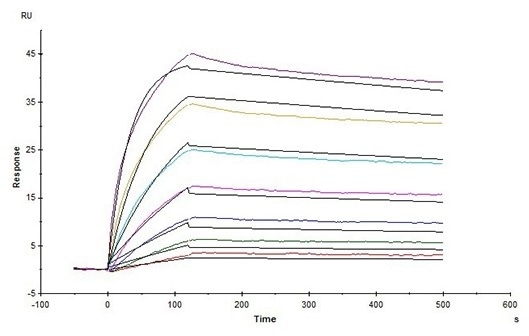
Human Claudin-18.2 Full Length Protein-VLP (Cat. No. CL2-H5547) captured on CM5 Chip via Anti-Claudin-18.2 antibody can bind Anti-Claudin-18.2 antibody with an affinity constant of 1.24 nM as determined in an SPR assay (Biacore T200) (Routinely tested). Image Credit: ACROBiosystems
Suitable for CAR detection
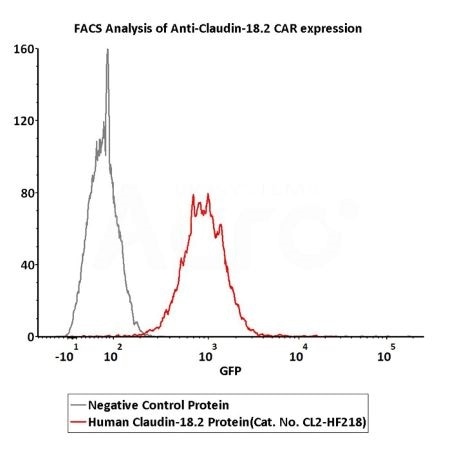
2e5 of Anti-Claudin-18.2 CAR-293 cells were stained with 100 μL of 3 μg/mL of Fluorescent Human Claudin-18.2 Full Length Protein-VLP (Cat. No.CL2-HF218) and negative control protein respectively, FITC signals was used to evaluate the binding activity (Routinely tested). Image Credit: ACROBiosystems
CD20-DDM/CHS
CD20-DDM/CHS. Image Credit: ACROBiosystems
Good bioactivity validation of full-length CD20-DDM/CHS (Cat. No.CD0-H52H3) by ELISA
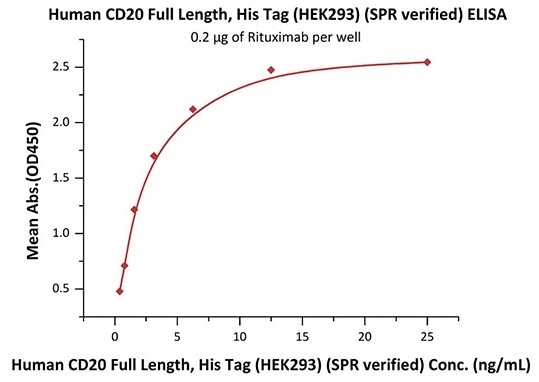
Immobilized Rituximab at 2 μg/mL (100 μL/well) can bind Human CD20 Full Length, His Tag, HEK293 (SPR verified) (Cat. No. CD0-H52H3) with a linear range of 0.4–3 ng/mL (in presence of DDM and CHS). Image Credit: ACROBiosystems
Good bioactivity validation of CD20-DDM/CHS (Cat. No.CD0-H82E5) by ELISA
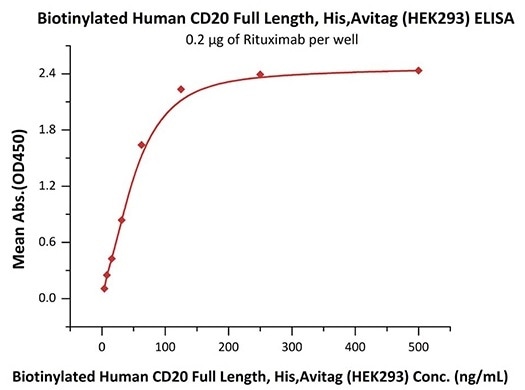
Immobilized Rituximab at 2 μg/mL (100 μL/well) can bind Biotinylated Human CD20 Full Length, His, Avitag (HEK293) (Cat. No. CD0-H82E5) with a linear range of 4–63 ng/mL (in presence of DDM and CHS). Image Credit: ACROBiosystems
High affinity validation of CD20-DDM/CHS (Cat. No.CD0-H82E5) by SPR
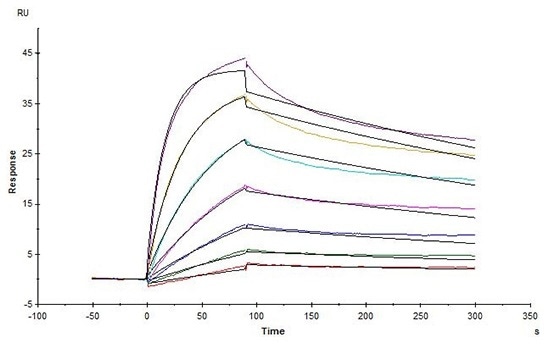
Biotinylated Human CD20, His, Avitag (HEK293) (Cat. No. CD0-H82E5) captured on Biotin CAP-Series S Sensor Chip can bind Rituximab with an affinity constant of 1.73 nM as determined in an SPR assay (in presence of DDM and CHS) (Biacore T200). Image Credit: ACROBiosystems
CD133-nanodisc
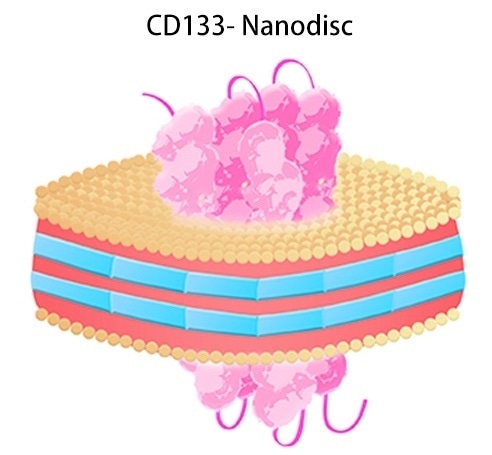
CD133-Nanodisc. Image Credit: ACROBiosystems
Purity greater than 90% by SDS-PAGE
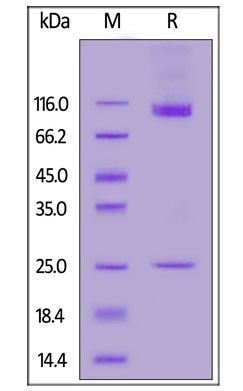
Human CD133 Full Length, His Tag (Nanodisc) (Cat. No. CD3-H52H1) on SDS-PAGE under reducing (R) condition. The gel was stained overnight with Coomassie Blue. The purity of the protein is greater than 90%. Image Credit: ACROBiosystems
Good bioactivity validated by ELISA
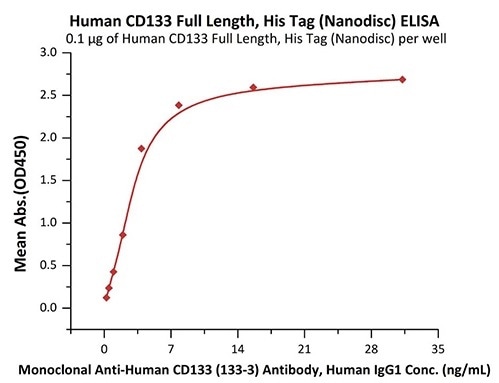
Immobilized Human CD133 Full Length, His Tag (Nanodisc) (Cat. No. CD3-H52H1) at 1 μg/mL (100 μL/well) can bind Monoclonal Anti-Human CD133 (133-3) Antibody, Human IgG1 with a linear range of 0.2–4 ng/mL (QC tested). Image Credit: ACROBiosystems