2. What should I know before I use EPREX®?
Do not use if you have ever had an allergic reaction to EPREX® or any of the ingredients
listed at the end of the CMI. Use of medicines like EPREX that stimulate red blood
cell production during chemotherapy has been associated with increased risk of death
in some studies. Your doctor should only use EPREX to treat your anaemia if it is
caused by chemotherapy and blood transfusions are not an appropriate treatment option.
Do not use if you have uncontrolled high blood pressure, been diagnosed with pure
red cell aplasia, cannot have transfusions with your own blood, or have severe heart
disease, disorders with your veins or arteries, or had a recent heart attack or stroke,
or are a surgery patient who should not be given medicines to thin your blood.
Talk to your doctor if you have any other medical conditions, take any other medicines,
or are pregnant or plan to become pregnant or are breastfeeding.
3. What if I am taking other medicines?
4. How do I use EPREX®?
5. What should I know while using EPREX®?
|
Things you should do
|
Remind any doctor, dentist or pharmacist you visit that you are using EPREX®. If you
are receiving dialysis treatment when you begin treatment with EPREX, your dialysis
regimen may need to be adjusted. Have regular blood tests and have blood pressure
taken when required by your doctor.
|
|
Things you should not do
|
Do not use EPREX® for any other complaint unless your doctor tells you to.
|
|
Driving or using machines
|
Be careful before you drive or use any machines or tools until you know how EPREX®
affects you.
EPREX® may cause dizziness in some people.
|
|
Looking after your medicine
|
Store EPREX between 2°C and 8°C in the refrigerator. Do not freeze and protect from
light.
|
6. Are there any side effects?
All medicines can have side effects. Sometimes they are serious, most of the time
they are not. If you notice any of the following, tell your doctor immediately as
you may need urgent medical care: severe, sudden, stabbing migraine-like headaches.
Seizures, confusion or epileptic fits. Raised blood pressure, clotting of your blood
in the haemodialysis system, or blockage of your fistula if you are on dialysis. Chest
pain, breathlessness, painful swelling in the leg. Signs of an allergic reaction like
skin rashes, hives, shortness of breath, wheezing or difficulty breathing, swelling
of the face or eyelids. Sudden tiredness, dizziness. For more information, including
what to do if you have any side effects, see Section
6. Are there any side effects? in the full CMI.
|
WARNING FOR CANCER PATIENTS: use of medicines like EPREX that stimulate red blood
cell production during chemotherapy has been associated with increased risk of death
in some studies. Your doctor should only use EPREX to treat your anaemia if it is
caused by chemotherapy and blood transfusions are not an appropriate treatment option.
|
Active ingredient(s): Epoetin alfa (rch)
Full Consumer Medicine Information (CMI)
This leaflet provides important information about using EPREX®. You should also speak to your doctor or pharmacist if you would like further information
or if you have any concerns or questions about using EPREX®.
Where to find information in this leaflet:
1. Why am I using EPREX®?
EPREX® contains the active ingredient epoetin alfa. EPREX® is a protein that stimulates bone marrow to produce more red blood cells.
Red blood cells are responsible for carrying oxygen to all parts of your body. A decrease
in the number of red blood cells can cause anaemia. Some symptoms of anaemia are tiredness,
breathlessness when exercising, and feeling cold. Anaemia may have many causes, including
decreased production of a hormone called erythropoietin by the kidneys due to kidney
failure, or as a result of chemotherapy treatments for cancer. EPREX is virtually
identical to your body's erythropoietin, and has a similar effect to naturally occurring
erythropoietin in your body.
EPREX® is used to treat the anaemia associated with kidney disease. If you have kidney
disease, your kidney may not produce enough erythropoietin (necessary for red blood
cell production) and your doctor may wish to correct this by prescribing EPREX. This
medicine stimulates your bone marrow to produce more red blood cells, helping to treat
your anaemia.
EPREX can also be used to treat anaemia if you are receiving chemotherapy for cancer
and your doctor decides that a blood transfusion is not appropriate.
Doctors can also prescribe EPREX for mildly anaemic patients who are
going to have surgery and donate blood before surgery, so that their own blood can
be given to them during or after surgery. Because EPREX stimulates the production
of red blood cells, a higher volume of blood can be taken from these patients.
EPREX can be used as an alternative to a blood transfusion in adult patients about
to undergo major orthopaedic (bone) surgery where there is a potentially high risk
from blood transfusion complications.
EPREX is not addictive.
This medicine is available only with a doctor's prescription.
2. What should I know before I use EPREX®?
Warnings
Do not use EPREX® if:
you are allergic to epoetin alfa, or any of the ingredients listed at the end of this
leaflet.
Always check the ingredients to make sure you can use this medicine.
If you have high blood pressure that is not properly controlled with blood pressure-lowering
drugs.
If you are a surgery patient who should not be given medicines to thin the blood.
If you are due to have elective surgery and you have severe heart disease, disorders
of the veins or arteries, or have recently had a heart attack or stroke.
If you have been diagnosed with Pure Red Cell Aplasia (your bone marrow cannot produce
enough red blood cells) after previous treatment with an erythropoietin product, including
EPREX.
If you cannot have transfusions with your own blood during or after surgery.
Do not use EPREX if the packaging is torn or shows signs of tampering.
Do not use EPREX beyond the expiry date (month and year) printed on the pack.
Do not misuse EPREX. Misuse by healthy people may lead to life-threatening problems with the heart and
blood vessels (e.g., stroke, heart attack, or blood clots).
Check with your doctor if you if you have or have had:
high blood pressure
heart disease (such as angina)
disorders of blood circulation resulting in pins and needles or cold hands or feet
or muscle cramps in the legs.
blood clotting disorders
seizures or epileptic fits take any medicines for any other condition
cancer. If you are a cancer patient be aware that erythropoietins like EPREX may act
as a growth factor and therefore in theory may affect the progression of your cancer.
Please discuss this with your doctor.
anaemia from other causes
liver disease
gout
porphyria (a rare blood pigment disorder)
an allergy to latex.
During treatment, you may be at risk of developing certain side effects. It is important
you understand these risks and how to monitor for them. See additional information
under Section
6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
In many women with severe kidney failure, their monthly periods may stop. In these
women, erythropoietin may restart the monthly cycle. Before starting EPREX, you should
discuss the need for contraception with your doctor.
Make sure you tell your doctor if you have any other medical problems since these
may affect the use of EPREX.
If you have used EPREX or another erythropoietin in the past, and you lost the good
response you were having, tell your doctor about this.
If you have not told your doctor or pharmacist about any of the above, tell them before
you start using or are given EPREX.
Your doctor will advise you whether or not to use EPREX or if you need to adjust the
dose or adapt your treatment.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any
medicines, vitamins or supplements that you buy without a prescription from your pharmacy,
supermarket or health food shop.
Iron is also a constituent of red blood cells. Therefore, iron supplements and other
blood stimulating drugs may increase your response to EPREX treatment. Your doctor
will decide whether you should take other medicines while using EPREX.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins
or supplements you are taking and if these affect EPREX®.
4. How do I use EPREX®?
How much to use
Your doctor will determine the correct dose of EPREX. EPREX injection is administered
either into a vein (intravenously) or just under the skin (subcutaneously). After
instruction, you can administer it under the skin yourself if you wish. Your doctor
can discuss with you whether injection into the vein or under the skin is preferable.
For patients with anaemia due to kidney failure, EPREX should be given intravenously,
(into a vein or a tube that goes into a vein) if intravenous access is routinely available
(haemodialysis patients). For patients not yet on dialysis and those on peritoneal
dialysis EPREX can be administered subcutaneously. The usual starting dose is 50 IU/kg
three times per week for adults and 25 IU/kg three times per week for children, after
which the dose may be changed by your doctor as needed.
For patients who are scheduled for surgery and who are not storing their own blood
the usual dose is 300 IU/kg body weight for 10 days before surgery, on the day of
surgery and for 4 days after. Alternatively a dose of 600 IU/kg may be administered
weekly for 3 weeks before surgery and on the day of surgery. The subcutaneous route
is used.
For anaemic cancer patients receiving chemotherapy, the initial dose is 150 IU/kg
three times per week. After 4 weeks your doctor will check your response and increase
the dose to 300 IU/kg three times weekly if response has been insufficient. If at
any stage EPREX has produced too many red cells, your doctor will stop the drug and
later re-start it at a lower dose. The subcutaneous route is used.
When to use EPREX®
Injecting EPREX under the skin yourself
At the start of your therapy, EPREX may be injected by medical or nursing staff. However,
your doctor may decide that it is right for you to learn how to inject EPREX under
the skin (subcutaneously) yourself. You will receive appropriate training for you
to do this. Under no circumstances should you attempt to inject yourself unless you
have been trained to do so.
Always use EPREX exactly as instructed by your doctor or nurse.
Only use EPREX if it has been stored correctly.
EPREX is for single use only.
EPREX should not be used, and should be discarded if:
the seal is broken,
the liquid is coloured or
particles are in it,
it may have been frozen, or
there has been a refrigeration failure.
Any unused product or waste material should be disposed of in accordance with local
requirements.
If EPREX is injected under the skin (subcutaneously), the amount injected is not normally
more than one millilitre (1 mL) in a single injection.
EPREX is given alone and not mixed with other liquids for injection.
Only use EPREX if the solution is clear and colourless.
Do not shake EPREX prefilled syringes.
Prolonged vigorous shaking may damage EPREX. If EPREX has been shaken vigorously,
don't use it.
How to inject EPREX under the skin
The pre-filled syringes are fitted with the PROTECS™ needle guard device to help prevent
needle stick injuries after use. This is indicated on the packaging.
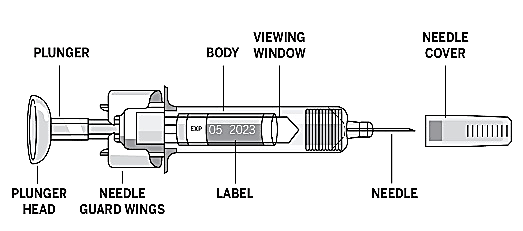
Take a syringe out of the refrigerator.
The liquid needs to come to room temperature. This usually takes between 15 to 30
minutes. Do not remove the syringe's needle cover while allowing it to reach room
temperature.
Check the syringe,
to make sure it is the right dose, has not passed its expiry date, is not damaged,
and the liquid is clear and not frozen.
Choose an injection site.
Good sites are the top of the thigh and around the tummy (abdomen) but away from the
navel. Vary the site from day to day.
Wash your hands. Use an antiseptic swab on the injection site, to disinfect it.
Hold the pre-filled syringe by the body of the syringe with the covered needle pointing
upward.
Do not hold by the plunger head, needle guard wings, or needle cover.
Do not pull back on the plunger at any time.
Do not remove the needle cover from the pre-filled syringe until you are ready to
inject your EPREX.
Take the needle cover off the syringe by holding the body and pulling the needle cover
off carefully without twisting it.
Don't push the plunger, touch the needle or shake the syringe.
Do not touch the needle guard wings to prevent prematurely covering the needle with
the needle guard.
Pinch a fold of skin
between your thumb and index finger. Don't squeeze it.
Push the needle in fully.
Push the plunger with your thumb as far as it will go to inject all of the liquid.
Push it slowly and evenly, keeping the skinfold pinched. The needle guard will not
activate unless the entire dose is given. You may hear a click when the needle guard
has been activated.
When the plunger is pushed as far as it will go,
take out the needle and let go of the skin.
Slowly take your thumb off the plunger.
Allow the syringe to move up until the entire needle is covered by the needle guard.
When the needle is pulled out of your skin, there may be a little bleeding at the
injection site.
This is normal. You can press an antiseptic swab over the injection site for a few
seconds after the injection.
Dispose of your used syringe
in a safe container.
Only take one dose of EPREX from each syringe. If any liquid remains in the syringe
after an injection, the syringe should be properly disposed of, not reused. EPREX
prefilled syringes do not contain preservatives.
Therefore, once a syringe has been opened, any remaining solution must be discarded.
If you do not understand the instructions provided with this medicine, ask your doctor
or pharmacist for help.
If you forget to use EPREX®
EPREX®should be used regularly at the same time.
If it is almost time for your next dose, skip the dose you missed and take your next
dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
If you use too much EPREX®
If you think that you have used too much EPREX®, you may need urgent medical attention.
You should immediately:
phone the Poisons Information Centre
(by calling
13 11 26), or
contact your doctor, or
go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using EPREX®?
Things you should do
Always follow your doctor's instructions carefully.
If you are receiving dialysis treatment when you begin treatment with EPREX, your
dialysis regimen may need to be adjusted. Your doctor will decide this.
You will need to have regular blood tests while you are using EPREX to see how you
respond to treatment with EPREX. Your doctor will order regular blood tests to ensure
that your medicine is continuing to work properly. If your haemoglobin levels are
above 120 g/L, discuss reducing your EPREX dose with your doctor.
Your doctor will need to monitor your blood pressure regularly, especially at the
beginning of treatment.
An increase in levels of small cells (called platelets) in your blood may occur, particularly
when starting haemodialysis treatment.
Tell your doctor if you become pregnant while using EPREX.
If you experience a severe skin reaction, a rash, which may be severe, may cover your
whole body and can also include blisters or areas of skin coming off, stop using EPREX
and call your doctor or get medical help right away.
If you are about to start taking a new medicine, tell your doctor and pharmacist that
you are using EPREX.
If you become increasingly tired, dizzy or breathless, you should talk to your doctor
at once. Your doctor can decide whether EPREX is not working properly for you and
will end the treatment if necessary.
If you are due to have major surgery, your doctor will give you a medicine to reduce
the risk of abnormal blood clotting.
Remember to tell your doctor if you received EPREX or another erythropoietin-like
medicine in the past and you experienced a worsening in your anaemia.
Take special care with other products that stimulate red blood cell production: EPREX
is one of a group of products that stimulate the production of red blood cells like
the human protein erythropoietin does. If you are given a product in this group other
than the one prescribed by your doctor during your treatment, speak to your doctor
before using it. It is important that you continue to use the same product in the
group unless your doctor says otherwise.
Remind any doctor, dentist or pharmacist you visit that you are using EPREX®.
Things you should not do
Do not use EPREX to treat any other complaint unless your doctor says so.
Do not give this medicine to anyone else, even if their symptoms seem similar to yours.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how EPREX®
affects you.
EPREX® may cause dizziness in some people
Looking after your medicine
Store EPREX between 2°C and 8°C in the refrigerator. Do not freeze and protect from
light. Immediately prior to use, EPREX may be stored in a room that stays below 25°C,
for a maximum single period of seven days.
Do not store EPREX, or any other medicine, in the bathroom or near a sink. Do not
leave medicines in the car or on windowsills. Heat and dampness can destroy some medicines.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy
for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of
them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you
have any further questions about side effects.
Less serious side effects
Serious side effects
Tell your doctor or pharmacist if you notice anything else that may be making you
feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can
report side effects to the Therapeutic Goods Administration online at
www.tga.gov.au/reporting-problems . By reporting side effects, you can help provide more information on the safety of
this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop
taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription. The prefilled syringes
are fitted with the PROTECS™ needle guard device to help prevent needle stick injuries
after use.
The needle cover of the prefilled syringe contains dry natural rubber (a derivative
of latex).
EPREX does not contain lactose or gluten.
What EPREX® contains
|
Active ingredient
(main ingredient)
|
Epoetin alfa (rch) (r-HuEPO).
|
|
Other ingredients
(inactive ingredients)
|
glycine (5 mg/mL)
polysorbate 80 (0.3 mg/mL)
sodium chloride at 1.7 - 5.8 mg, monobasic sodium phosphate dihydrate at 0.35 - 1.16
mg, dibasic sodium phosphate dihydrate at 0.67 - 2.22 mg, sodium citrate at less than
5 mmol and water for injections.
|
|
Potential allergens
|
latex
|
Do not take this medicine if you are allergic to any of these ingredients.
What EPREX® looks like
EPREX injection is a clear, colourless solution in prefilled syringes of 1000 IU in
0.5 mL, 2000 IU in 0.5 mL, 3000 IU in 0.3 mL, 4000 IU in 0.4 mL, 5000 IU in 0.5 mL,
6000 IU in 0.6 mL, 8000 IU in 0.8 mL, 10000 IU in 1.0 mL, 20,000 IU in 0.5 mL, 30,000
IU in 0.75 mL and 40,000 IU in 1.0 mL. Each box contains 6 prefilled syringes (1 syringe
for 40,000 IU).
Australian Registration numbers:
Phosphate buffered syringes:
EPREX 1000 0.5 mL AUST R 65442
EPREX 2000 0.5 mL AUST R 65443
EPREX 3000 0.3 mL AUST R 65444
EPREX 4000 0.4 mL AUST R 65445
EPREX 5000 0.5 mL AUST R 76970
EPREX 6000 0.6 mL AUST R 76971
EPREX 8000 0.8 mL AUST R 76973
EPREX 10000 1.0 mL AUST R 65446
EPREX 20000 0.5 mL AUST R 73486
EPREX 30000 0.75 mL AUST R 135069
EPREX 40000 1.0 mL AUST R 73487
Who distributes EPREX®
JANSSEN-CILAG Pty Ltd
1-5 Khartoum Road
Macquarie Park NSW 2113 Australia
Telephone: (02) 8875 3333
NZ Office: Auckland New Zealand
Telephone: 0800 800 80
This leaflet was prepared in November 2025