This article and associated images are based on a poster originally authored by Charachakorn Eurtivong, Euphemia Leung, Ivanhoe Leung and Jóhannes Reynisson and presented at ELRIG Drug Discovery 2025 in affiliation with Mahidol University, University of Auckland, The University of Melbourne and Keele University.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Introduction
Phosphatidylcholine-specific phospholipase C (PC PLC) is an enzyme that catalyzes the formation of the important secondary messengers phosphocholine (PC) and diacylglycerol (DAG) from phosphatidylcholine, secondary cell signal messengers (Fig. 1).
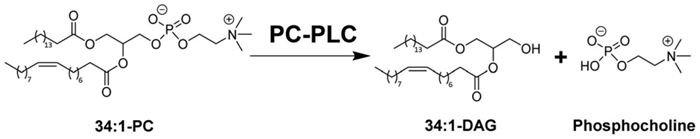
Figure 1. PC-PLCBc is a zinc-dependent enzyme that catalyses the hydrolysis of PC into phosphocholine and DAG. Image Credit: Charachakorn Eurtivong et al., in partnership with ELRIG (UK) Ltd.
Although PC-PLC has been linked to the progression of many pathological conditions, including cancer, atherosclerosis, inflammation, and neuronal cell death, studies on PC-PLC are relatively scarce. To date, the human gene expressing PC-PLC has not yet been found, and the only protein structure is from Bacillus cereus (PC-PLCBc).1
A handful of inhibitors have been developed against PC-PLC, but none of them are drug-like, e.g., tricyclodecan-9-yl-xanthogenate (D609).
The aim of this project was to develop drug-like lead series by:
- Structure based virtual screening
- SAR development of the hits
- Development of an improved binding assay.
Virtual screening
The ChemBridge diversity library (5 × 105) was screened against the PC-PLCBc binding pocket using GOLD (Fig. 2).2
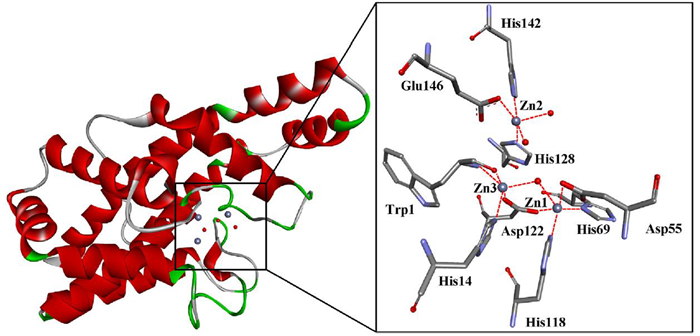
Figure 2. The PC-PLCBc structure (PDB: 1AH7). It has three catalytic Zn2+ ions and water (grey and red spheres) in the binding pocket. Image Credit: Charachakorn Eurtivong et al., in partnership with ELRIG (UK) Ltd.
Assays
The Amplex Red biochemical assay was used to test the virtual hits. IC50 values were derived for the best hits, and these were verified using the intrinsic tryptophan fluorescence assay (KD). The best hit series was of 2-Morpholinobenzoic acids (84).
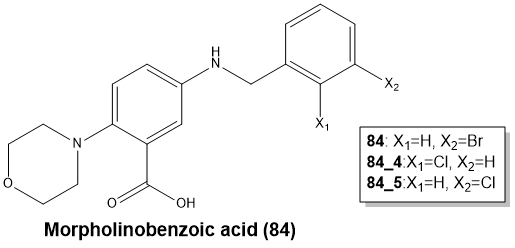
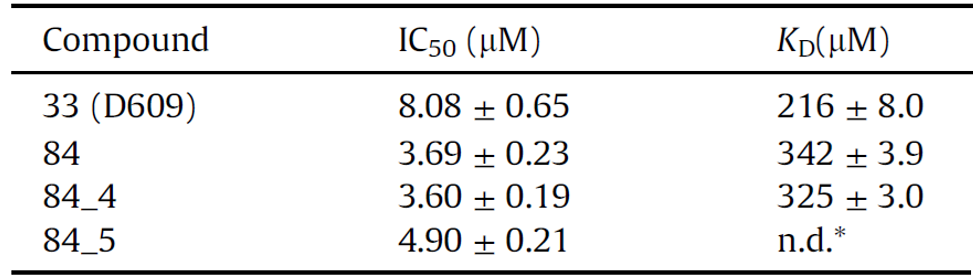
Image Credit: Charachakorn Eurtivong et al., in partnership with ELRIG (UK) Ltd.
SAR generation
129 derivatives of compound 84 were synthesized, focusing on three parts of the molecule (Fig. 3).
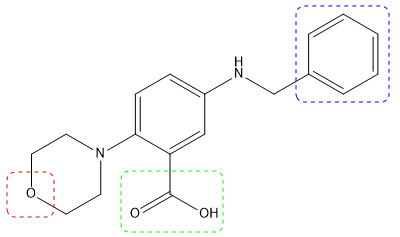
Figure 3. Hit compound 84 and points of variation explored: morpholine, carboxylic acid, and phenyl ring. Image Credit: Charachakorn Eurtivong et al., in partnership with ELRIG (UK) Ltd.
The morpholine shows the best activity compared to piperidine and piperazine. Removal of the carboxylic acid was beneficial and ortho substitution on the phenyl ring with small groups was favorable.3 Also, hydroxyamic acid chelates strongly with zinc ions and was substituted for the carboxylic acid moiety. No improvement was observed in activity inhibition.4
MALDI-TOF assay
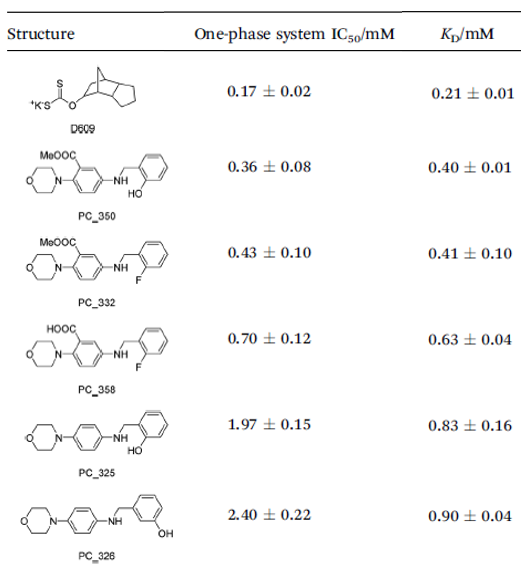
To improve the reliability of the SAR study, an improved assay was developed and compared to the intrinsic tryptophan fluorescence assay, resulting in similar values (Table 1).5
Table 1. The activity and binding values of the inhibitors. Source: ELRIG (UK) Ltd.
Conclusion
Structurally based virtual screening was conducted against the PC-PLCBc. 5 × 105 compounds were screened, resulting in seven hits. SAR studies and testing using the intrinsic tryptophan fluorescence assay for ligand-protein binding resulted in the 2-Morpholinobenzoic acid series having the best inhibition.
The SAR of this series was further explored, confirming that the morpholine moiety is optimal, carboxylic acid is not necessary for binding, and the ortho position on the phenyl ring is favorable.
The new MALDI-TOF assay yielded similar results, except for the effect of carboxylic acids and their derivatives. In all, a new inhibitor series was developed demonstrating that PC PLC can be modulated with drug-like ligands.
References
- Chatchakorn Eurtivong, et al. (2023). Phosphatidylcholine-Specific Phospholipase C as a Promising Drug Target. Molecules, 28(15), pp.5637–5637. https://doi.org/10.3390/molecules28155637.
- Eurtivong et al. Eur. J. Med. Chem. Chem., 2020, 187, 111919.
- Pilkington, L.I., et al. (2020). Development, synthesis and biological investigation of a novel class of potent PC-PLC inhibitors. European Journal of Medicinal Chemistry, [online] 191, p.112162. https://doi.org/10.1016/j.ejmech.2020.112162.
- Rees, et al. Bioorg. Chem. Chem., 2021, 114, 105152.
- Sharma, et al. Anal. Meth. Meth., 2021, 13, 491.
About Keele University
Keele University is a public research university based in Staffordshire, England. The institution was founded in 1949 as the University College of North Staffordshire and received its Royal Charter to become Keele University in 1962.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free to attend!
Our values
Our values are to always ensure the highest quality of content, that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer-led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world-class conferences, networking events, webinars, and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.