2. What should I know before I use HYQVIA?
Do not use HYQVIA if you have severe immunoglobulin A (IgA) deficiency and history
of hypersensitivity to human immunoglobulin treatment. Do not use if you have ever
had an allergic reaction to HYQVIA or any of the ingredients listed at the end of
the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines,
or are pregnant or plan to become pregnant or are breastfeeding. For more information, see Section
2. What should I know before I use HYQVIA? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with HYQVIA and affect how it works.
4. How do I use HYQVIA?
HYQVIA should be used exactly as your doctor has told you.
HYQVIA must be infused under the skin.
5. What should I know while using HYQVIA?
|
Things you should do
|
Remind any doctor, dentist or pharmacist you visit that you are using HYQVIA.
Talk to your healthcare provider before travelling. It is important to obtain a written
statement from your physician, explaining the reasons why you need to have this medicine
and injecting devices with you, otherwise you may not be allowed to bring it into
the country of travelling.
|
|
Things you should not do
|
Do not stop using this medicine or lower the dosage, without checking with your doctor,
unless you have an allergic reaction.
|
|
Driving or using machines
|
HYQVIA has no, or negligible, influence on the ability to drive or use machines.
If you experience adverse reactions, such as dizziness and nausea, you should wait
for these to resolve before driving or operating machines.
|
|
Looking after your medicine
|
Store at 2°C - 8°C in a refrigerator for the duration of its shelf life. Do not freeze.
Store in the original package to protect from light.
|
6. Are there any side effects?
Common side effects include decreased appetite, migraine, headache, dizziness, burning
sensation, hypertension, blood pressure decreased, nasal congestion, nausea, abdominal
pain, vomiting, diarrhoea, chest pain, oral pain, infusion site swelling, redness,
or itching, fever or chills, back and muscle pain or joint pain, fatigue, swelling
of hands, ankles or feet unusual weakness, feeling abnormal. For more information,
including what to do if you have any side effects, see Section
6. Are there any side effects? in the full CMI.
This medicine is subject to additional monitoring due to approval of an extension
of indications. This will allow quick identification of new safety information. You
can help by reporting any side effects you may get. You can report side effects to
your doctor, or directly at www.tga.gov.au/reporting-problems.
Active ingredient(s):
normal immunoglobulin 10% (Human) with vorhyaluronidase alfa
Full Consumer Medicine Information (CMI)
This leaflet provides important information about using HYQVIA. You should also speak to your doctor or pharmacist if you would like further information
or if you have any concerns or questions about using HYQVIA.
The information in this leaflet was last updated on the date listed in 'Section 7.
Product details'. More recent information on the medicine may be available. You should
ensure that you speak to your pharmacist or doctor to obtain the most up to date information
on this medicine.
Where to find information in this leaflet:
1. Why am I using HYQVIA?
HYQVIA contains the active normal immunoglobulin 10% (Human) with vorhyaluronidase
alfa.
HYQVIA belongs to a class of medicines called "human immunoglobulins". Immunoglobulins
are also known as antibodies and are found in healthy people's blood. Antibodies are
part of the immune system (the body's natural defenses) and help your body to fight
infections. If you do not have enough antibodies, you may not be able to fight off
infections.
HYQVIA is used in patients who do not have enough antibodies in their blood. HYQVIA can be used as
antibody replacement therapy to raise antibody levels in your blood to normal levels.
HYQVIA is also used in patients with chronic inflammatory demyelinating polyneuropathy (CIDP), a form
of autoimmune disease. CIDP is characterised by chronic inflammation of the peripheral
nerves that causes muscle weakness and/or numbness mainly in the legs and arms. It
is believed that the body’s own defense system attacks the peripheral nerves and causes
nerve damage and inflammation. Immunoglobulins present in HYQVIA are thought to help
protect the nerves from being damaged by the immune system.
HYQVIA can be used in adults and children 2 years of age and older.
2. What should I know before I use HYQVIA?
Warnings
About blood products
When medicines are made from human blood or plasma, processes are used to prevent
infections being passed from the blood/plasma donor to the person receiving the medicine.
These processes include careful selection of the people who donate blood and plasma
to make sure that those who might be carrying infections are excluded. In addition,
each donation and pools of donations are tested for indicators of virus or virus infection(s).
Manufacturers of these medicines also include steps in the processing of blood or
plasma that inactivate or remove viruses. A three-step viral inactivation/reduction
has been applied during the manufacturing of the Normal Immunoglobulin Infusion. Despite
the stringent measures, which have been put in place during the manufacturing processes,
the risk of contamination by viral and other unknown agents cannot be totally excluded.
The measures taken during manufacturing are considered effective for enveloped viruses
such as human immunodeficiency virus (HIV), hepatitis B virus and hepatitis C virus,
and for the non-enveloped viruses hepatitis A (HAV) and B19 virus (B19V).
Immunoglobulins have not been associated with hepatitis A or parvovirus B19 infections
possibly because the antibodies against these infections, which are contained in the
product, are protective.
HYQVIA should not be used if
You are allergic to immunoglobulins, hyaluronidase, vorhyaluronidase alfa or are allergic
to any ingredients in HYQVIA (see "Product Description").
Do not use HYQVIA if:
you have had a history of anaphylactic or severe systemic reactions to the administration
of IgG
you have antibodies against immunoglobulin A (IgA) in your blood. This may occur if
you have IgA deficiency
you have systemic hypersensitivity to hyaluronidase or vorhyaluronidase alfa, or any
of the other excipients
have allergies to any other medicines, or if you have ever had an allergic reaction
to an injection
Always check the ingredients to make sure you can use this medicine.
Check with your doctor if you:
have any other medical problems
have had a vaccination recently.
Your doctor may request regular monitoring of your kidney function after you started
receiving HYQVIA.
During treatment, you may be at risk of developing certain side effects. It is important
you understand these risks and how to monitor for them. See additional information
under Section
6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any
medicines, vitamins or supplements that you buy without a prescription from your pharmacy,
supermarket or health food shop.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins
or supplements you are taking and if these affect HYQVIA.
4. How do I use HYQVIA?
Always use HYQVIA exactly as your doctor has told you. Check with your doctor if you
are not sure
HYQVIA must be infused under the skin
Treatment with HYQVIA will be started by your doctor or nurse, but you may be allowed
to use the medicine at home once you have received the first few infusions under medical
supervision and you (and/or your guardian) have been adequately trained. You and your
doctor will decide if you can use HYQVIA at home. Do not begin treatment with HYQVIA
at home until you have received complete instructions
Always wash your hands before doing the following procedures. Use germ-free methods
during the making up procedure and during injection
HYQVIA must not be mixed with other injectable medicines
Do not inject HYQVIA near an infected or inflamed area of the body
Follow the instructions provided and do not stop using HYQVIA until your doctor tells
you to stop.
How to use HYQVIA
If you do not understand the instructions, ask your doctor or health professional.
Always follow the specific instructions given by your healthcare provider. The steps
listed below are general guidelines for using your medicine.
For the ease of identification, the vorhyaluronidase alfa (Hyaluronidase) vial is
labelled HY and the Normal Immunoglobulin Infusion 10% (human) vial is labelled IG.
Instructions for use
If you forget to use HYQVIA
Do not infuse a double dose of HYQVIA to make up for a missed dose. If you think that
you have missed a dose speak to your doctor as soon as possible.
If you use too much HYQVIA
The effects of an overdose of HYQVIA are not known. Please tell your doctor if you
accidently use more than instructed.
5. What should I know while using HYQVIA?
Things you should do
Stop the infusion immediately and contact your doctor, if you experience allergic
reactions such as skin rash, itching, chest tightness, wheezing, dizziness, hives,
faintness, chills, flushing, rapid heartbeat, shortness of breath and/or a swollen
face
Always follow your doctor's instructions carefully
Tell all the doctors, dentists and pharmacists who are treating you that you are using
HYQVIA
If you are about to be started on any new medicine, tell your doctor and pharmacist
that you are using HYQVIA
If you become pregnant while you are using your medicine, tell your doctor
Talk to your healthcare provider before travelling. Plan to bring enough medicine
for your treatment during this time. It is important to obtain a written statement
from your physician, explaining the reasons why you need to have this medicine and
injecting devices with you, otherwise you may not be allowed to bring it into the
country of travelling.
Things you should not do
Do not give your medicine to anyone else, even if they have the same condition as
you
Do not use your medicine to treat any other complaints unless your doctor tells you
to
Do not stop using your medicine or lower the dosage, without checking with your doctor,
unless you have an allergic reaction.
Driving or using machines
HYQVIA has no, or negligible, influence on the ability to drive or use machines.
If you experience adverse reactions, such as dizziness and nausea, you should wait
for these to resolve before driving or operating machines.
Looking after your medicine
HYQVIA should be stored at 2°C - 8°C in a refrigerator for the duration of its shelf
life. Store in the original package to protect from light.
Do not freeze.
Keep out of the reach and sight of children.
Do not use HYQVIA after the expiry date which is printed on the label after the word
'EXP'.
The expiry date refers to the last day of that month.
Getting rid of any unwanted medicine
HYQVIA contains no preservatives.
Discard any medicine left in the vials at the end of your infusion.
Dispose of all materials, including any leftover reconstituted medicine, in an appropriate
container.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist
how to dispose of medicines no longer required. These measures will help to protect
the environment.
6. Are there any side effects?
Like all medicines, this medicine can have side effects, although not everybody gets
them. Certain side effects, such as headache, chills, or body aches, may be reduced
by slowing the infusion rate.
See the information below and, if you need to, ask your doctor or pharmacist if you
have any further questions about side effects.
Less serious side effects
Serious side effects
|
Serious side effects
|
What to do
|
|
Serious allergic reaction: hives, swelling in the mouth or throat, itching, trouble
breathing, wheezing, fainting or dizziness.
Swelling of the brain: bad headache with nausea, vomiting, stiff neck, fever, and
sensitivity to light
Kidney problem: reduced urination, sudden weight gain, or swelling in your legs
Chest pain or tightness
Pain or tenderness, redness or swelling in the arms and legs, other than at the infusion
sites
Blood clot: Pain, swelling, warmth, redness, or a lump in your legs or arms, other
than at the infusion site(s).
Liver or blood problem: brown or red urine, fast heart rate, yellow skin or eyes
Lung problem: chest pain or trouble breathing, blue lips or extremities
Lightheadedness
Fast heart rate
Abnormal heart rate, blueness of lips or fingers and toes
Blurred vision
Abdominal pain
Pain in the joints or extremity
|
Call your doctor straight away or go straight to the Emergency Department at your
nearest hospital if you notice any of these serious side effects.
|
Tell your doctor or pharmacist if you notice anything else that may be making you
feel unwell.
Other side effects not listed here may occur in some people. You can ask your doctor
or pharmacist for information that is written for healthcare professionals.
Reporting side effects
After you have received medical advice for any side affects you experience, you can
report side effects to the Therapeutic Goods Administration online at
www.tga.gov.au/reporting-problems . By reporting side effects, you can help provide more information on the safety of
this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop
taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
HYQVIA is a dual vial unit consisting of one vial of Normal Immunoglobulin Infusion
10% (Human) and one vial of vorhyaluronidase alfa.
HYQVIA is a dual vial unit containing:
1. a solution of vorhyaluronidase alfa (Step 1 of HYQVIA/Infuse first) and
2. a solution of Normal Immunoglobulin Infusion 10% (Human) (Step 2 of HYQVIA/Infuse
second).
What HYQVIA contains
|
Active ingredients
(main ingredients)
|
Normal Immunoglobulin 10% (Human) Infusion
Vorhyaluronidase alfa
|
|
Other ingredients
(inactive ingredients)
|
Glycine, water for injection
Dibasic sodium phosphate dihydrate, sodium hydroxide, human albumin 25%, calcium chloride,
sodium chloride, disodium edetate, water for injection
|
Do not take this medicine if you are allergic to any of these ingredients.
What HYQVIA looks like
The Normal Immunoglobulin Infusion is a clear or slightly opalescent and colourless
or pale-yellow solution. Vorhyaluronidase alfa is a clear, colourless solution.
The following pack sizes are available:
Vorhyaluronidase alfa - 1.25mL (volume); Normal immunoglobulin 10% (Human) - 2.5g
(protein) 25mL (volume)
Vorhyaluronidase alfa - 2.5mL (volume); Normal immunoglobulin 10% (Human) - 5g (protein)
50mL (volume)
Vorhyaluronidase alfa - 5mL (volume); Normal immunoglobulin 10% (Human) - 10g (protein)
100mL (volume)
Vorhyaluronidase alfa - 10mL (volume); Normal immunoglobulin 10% (Human) - 20g (protein)
200mL (volume)
Vorhyaluronidase alfa - 15mL (volume); Normal immunoglobulin 10% (Human) - 30g (protein)
300mL (volume)
Not all pack sizes may be marketed.
AUST R 235178
Who distributes HYQVIA
Takeda Pharmaceuticals Australia Pty Ltd
Level 39, 225 George Street
Sydney NSW 2000
Australia
Telephone: 1800 012 612
This leaflet was prepared in September 2025.
8. Instructions for use
1. Remove HYQVIA from the box:
Allow vials to reach room temperature. This may take up to 60 minutes. Do not use
heating devices including microwave.
Do not heat up or shake HYQVIA.
Check each vial of HyQvia before using:
Expiry date: Do not use beyond expiration date.
Colour:
The vorhyaluronidase alfa should be clear and colourless.
The human normal immunoglobulin 10% should be clear and colourless or pale yellow.
If either liquid is cloudy or has particles, do not use.
Cap: protective cap is on the dual vial unit. Do not use the product if it does not have
the cap.
2. Gather all supplies:
Collect all items for your infusion. Items include dual vial unit(s) of HYQVIA, infusion
supplies (subcutaneous needle set, solution container (bag or syringe), sterile clear
bandage and tape, pump tubing, transfer devices, syringes, gauze and tape), sharps
container, pump, and treatment logbook and other supplies as needed.
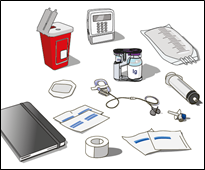
3. Prepare a clean work area.
4. Wash hands:
Wash your hands thoroughly. Place all gathered supplies and open them as directed
by your healthcare professional.
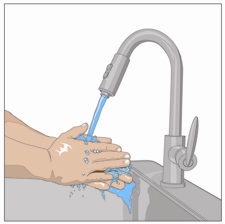
5. Open HYQVIA dual vial unit(s):
Remove protective cap(s) and make sure the blue vial caps are removed. If not, manually
remove the blue caps to expose the vial stoppers.
Prepare to transfer the vorhyaluronidase alfa component of HYQVIA by wiping each vial
stopper with an alcohol swab, if directed and allow to air dry (at least 30 seconds).
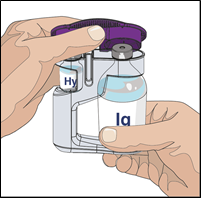
6. Prepare vorhyaluronidase alfa vial (HY):
Remove the smaller sterile syringe from package and attach it to a non-vented spike
or needle (device).
Pull back on the plunger, fill the smaller syringe with air equal to the amount of
vorhyaluronidase alfa in the HY vial(s).
Remove the cap of needle/non-vented transfer device.
Insert the tip of the needle/non-vented transfer device into the centre of the vial
stopper and push straight downward. Push the air into the vial.
Turn the vial upside down, with the needle/non-vented transfer device remaining in
the vial. The syringe tip will be pointing upward.
Withdraw the full contents of the vorhyaluronidase alfa into the syringe.
Repeat Step 6, if more than one vial of vorhyaluronidase alfa is needed for your dose.
If possible, combine all of the vorhyaluronidase alfa needed for the entire dose of
IgG into the same syringe.
Point the syringe tip up and remove any air bubbles by pointing the syringe tip up
and gently tapping the syringe with your finger. Slowly and carefully push the plunger
to remove any remaining air.
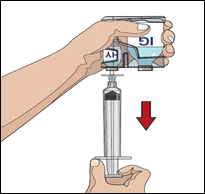
7. Prepare the needle set with the vorhyaluronidase alfa (HY):
IF using the push method to deliver (HY):
Attach the syringe filled with vorhyaluronidase alfa to the needle set
Push the plunger of smaller syringe to remove the air and fill the needle set up to
the needle wings with the vorhyaluronidase alfa.
Note: Your healthcare professional may recommend using a "Y" connector (for more than
one site) or other needle set configuration.
IF using the pump method to deliver (HY):
Attach the syringe filled with vorhyaluronidase alfa (HY) to the pump tubing and attach
the needle set
Push the plunger of syringe (size may vary due to a larger volume) to remove the air
and fill the pump tubing and needle set up to the needle wings with the vorhyaluronidase
alfa.
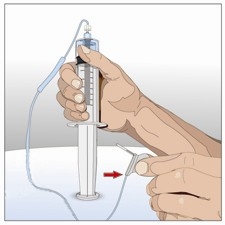
8. Prepare human normal immunoglobulin 10% vial:
Prepare to transfer the immunoglobulin 10% component of HYQVIA by wiping each vial
stopper with a separate alcohol swab, if directed and allow to air dry (at least 30
seconds).
The human normal immunoglobulin 10% of HYQVIA may be infused either:
by pooling from the vials either into larger syringe (a) or an infusion bag (b) as
directed by your healthcare professional, depending upon the pump to be used; or
directly from the IG vial. Insert the spike of the vented pump tubing or spike and
venting needle into human normal immunoglobulin 10% vial(s). Fill the administration
pump tubing and set aside until the recombinant human hyaluronidase has been administered.
If more than one vial is required for a full dose, spike subsequent vials after the
first vial has been fully administered.
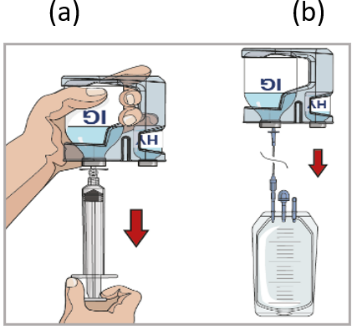
9. Prepare the pump:
Follow the manufacturer’s instructions for preparing the pump.
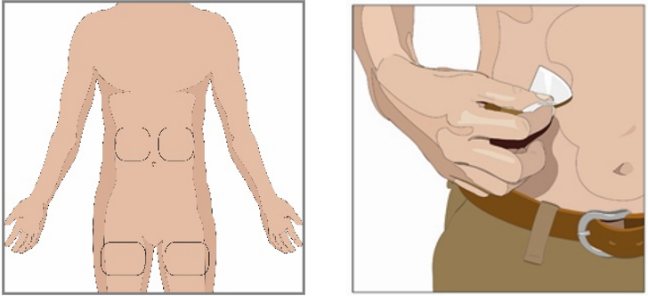
10. Prepare the infusion site:
Choose an infusion site(s) in either the middle to upper abdomen or thigh. See image
for infusion site locations.
Select sites on the opposite sides of the body if instructed to infuse in two sites
for doses above 600 mL.
If using three sites, the sites should be 10 cm apart
Avoid bony areas, visible blood vessels, scars and any areas of inflammation or infection.
Rotate infusion sites by choosing opposite sides of the body between future infusions.
If instructed by your health care professional, clean the infusion site(s) with an
alcohol swab. Allow to dry (at least 30 seconds).
11. Insert the needle:
Remove the needle cover. Firmly grasp and pinch at least 2 to 2.5 cm of skin between
two fingers.
Insert needle completely to the wings of the needle with a rapid motion straight into
the skin at a 90-degree angle. Wings of needle should lay flat on the skin.
Secure needle in place with sterile tape.
Repeat this step if you have a second or third infusion site.
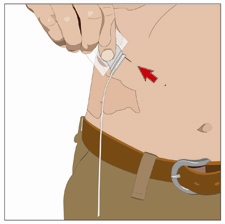
90-degree angle to skin
12. Check for proper needle placement before starting the infusion if instructed by
your healthcare professional.
13. Secure the needle to the skin:
Secure the needle(s) in place by putting a sterile clear bandage over the needle.
Check infusion site(s) occasionally throughout the infusion for dislodgement or leaking.
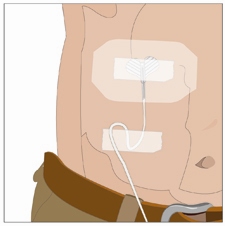
14. Administer the vorhyaluronidase alfa infusion first:
Divide the contents equally between all sites, if more than one site is used.
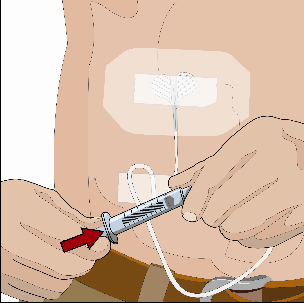
If using the push method to deliver HY:
Slowly push the plunger of the smaller syringe with the vorhyaluronidase alfa at an
initial rate per infusion site to approximately 1 to 2 mL per minute and increase
as tolerated.
If using the pump method to deliver HY:
If using a pump, prepare the pump to infuse the vorhyaluronidase alfa at an initial
rate per infusion site of 60 to 120 mL/hour/site and increase as tolerated.
15. Administer the human normal immunoglobulin 10%:
After infusing all of the content of the smaller syringe (vorhyaluronidase alfa),
remove the syringe from the hub of the needle set/pump tubing.
Attach the pump tubing to the IG container/vial or, the larger syringe containing
human normal immunoglobulin 10% to the needle set.
Administer the human normal immunoglobulin 10% with a pump at the rates prescribed
by your healthcare professional and start the infusion.
16. Flush the pump tubing when the infusion is complete if instructed by your healthcare
professional:
If instructed by your healthcare professional, attach a sodium chloride solution bag
to the pump tubing/needle set to push the human normal immunoglobulin 10% up to the
needle wings.
17. Remove needle set:
Remove the needle set by loosening the dressing on all edges.
Pull the needle wings straight up and out.
Gently press a small piece of gauze over the needle site and cover with a protective
dressing.
Throw away the needle(s) into the sharps container.
Dispose of the sharp's container using instructions provided with the container or
contact your healthcare professional.
18. Record the infusion:
Remove the peel-off label from HYQVIA vial, which has the product lot number and expiry
date and place the label in your treatment record/logbook.
Write down the date, time, dose, site(s) of infusion (to assist in rotating sites)
and any reactions after each infusion.
Throw away any unused product in the vial and the disposable supplies as recommended
by your healthcare professional.
Follow up with physician as directed.
HYQVIA® is a registered trademark of Baxalta Incorporated.
TAKEDA® and the TAKEDA logo® are registered trademarks of Takeda Pharmaceutical Company
Limited.