2. What should I know before I use UPTRAVI?
Do not use if you have ever had an allergic reaction to selexipag or any of the ingredients
listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines,
or are pregnant or plan to become pregnant or are breastfeeding.
3. What if I am taking other medicines?
Some medicines may interfere with UPTRAVI and affect how it works.
4. How do I use UPTRAVI?
At the start of treatment, you will take the lowest dose. This is one 200 micrograms
tablet in the morning and another 200 micrograms tablet in the evening. As instructed
by your doctor, you will gradually increase your dose. This is called titration. It
lets your body adjust to the new medicine. The goal of titration is to reach the highest
dose you can tolerate. This can be up to a maximum dose of 1600 micrograms twice a
day.
Swallow the tablets whole, do not split, crush or chew the UPTRAVI tablets.
5. What should I know while using UPTRAVI?
|
Things you should do
|
Remind any doctor or pharmacist you visit that you are taking UPTRAVI.
Tell your doctor if you become pregnant or are trying to become pregnant.
Tell your doctor if, for any reason, you have not used your medicine exactly as prescribed.
Keep all of your doctor or clinic appointments.
|
|
Things you should not do
|
Do not stop using this medicine unless you have agreed this with your doctor.
|
|
Driving or using machines
|
Be careful driving or operating machinery until you know how UPTRAVI affects you.
If you are affected, do not drive or operate machinery.
|
|
Looking after your medicine
|
Store below 30°C. Protect from moisture.
|
6. Are there any side effects?
The most common side effects are headache, diarrhoea, feeling sick, being sick, jaw
pain, muscle pain, pain in hands, arms, legs or feet, flushing, and joint pain. Serious
side effects are allergic reaction or anaphylaxis and kidney failure.
Active ingredient(s): Selexipag (se-le-xi-pag)
Full Consumer Medicine Information (CMI)
This leaflet provides important information about using UPTRAVI. You should also speak to your doctor or pharmacist if you would like further information
or if you have any concerns or questions about using UPTRAVI.
Where to find information in this leaflet:
1. Why am I using UPTRAVI?
UPTRAVI contains the active ingredient selexipag. UPTRAVI is a medicine that acts on a receptor that is the target of a natural substance
called "prostacyclin".
UPTRAVI is used to treat pulmonary arterial hypertension (PAH) in adults.
It can be used on its own or with other medicines for PAH. PAH is high blood pressure
in the blood vessels that carry blood from the heart to the lungs (the pulmonary arteries).
In people with PAH, these arteries narrow, so the heart has to work harder to pump
blood through them. This may cause people to feel tired, dizzy, short of breath, or
experience other symptoms.
UPTRAVI widens the pulmonary arteries and reduces their hardening. This makes it easier
for the heart to pump blood through the pulmonary arteries. It relieves the symptoms
of PAH and improves the course of the disease.
2. What should I know before I use UPTRAVI?
Warnings
Do not use UPTRAVI if:
you are allergic to selexipag, or any of the ingredients listed at the end of this
leaflet.
you have severe liver problems (Child-Pugh Class C)
you have had a stroke within the last 3 months
you have severe coronary heart disease or unstable angina
you have had a myocardial infarction (heart attack) within the last 6 months
you have a weak heart (decompensated cardiac failure) that is not under close medical
observation
you have severe arrhythmias (irregular heartbeat problem)
you have defect of your heart valves (inborn or acquired) that causes the heart to
work poorly (not related to pulmonary hypertension)
you are taking medicines that are strong inhibitors of CYP2C8 (e.g. gemfibrozil).
Check with your doctor if you:
have low blood pressure or experience dizziness or fainting
have problems with your liver
have severe problem with your kidneys or undergoing dialysis
have problems with thyroid gland
During treatment, you may be at risk of developing certain side effects. It is important
you understand these risks and how to monitor for them. See additional information
under Section
6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant or if you
are breastfeeding or intend to breastfeed.
UPTRAVI is not recommended during pregnancy and breastfeeding. There is no experience
with the use of this medicine during human pregnancy.
Women with PAH should avoid becoming pregnant as your condition may worsen. If you
are a woman who can have children, you should use an effective contraceptive method
while taking UPTRAVI.
Children and adolescents
Do not use UPTRAVI in children under 18 years of age. There is no experience with
the use of this medicine in children or adolescents under 18 years old.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any
medicines, vitamins or supplements that you buy without a prescription from your pharmacy,
supermarket or health food shop.
You must tell your doctor if you are taking medicines for high blood pressure.
You must tell your doctor if you are taking:
Rifampicin (antibiotic used to treat infections)
Sodium valproate or valproic acid (used to treat epilepsy)
Gemfibrozil (medicine used to lower the levels of fat in the blood)
Lopinavir and ritonavir (antivirals used to treat HIV)
Clopidogrel (used to treat heart disease/strokes)
Deferasirox (used to treat some blood disorders)
Teriflunomide (used to treat nervous system disorders)
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins
or supplements you are taking and if these affect UPTRAVI.
4. How do I use UPTRAVI?
How much to take
Your doctor will decide what dose of UPTRAVI is suitable for you.
Always take UPTRAVI exactly as your doctor has told you. Check with your doctor if
you are not sure.
Tell your doctor if you have problems with your liver or are taking other medications
as your doctor may recommend different dose of UPTRAVI.
Finding the right dose for you
At the start of treatment, you will take the lowest dose. This is one 200 micrograms
tablet in the morning and another 200 micrograms tablet in the evening. It is recommended
to take the first dose in the evening. As instructed by your doctor, you will gradually
increase your dose. This is called titration. It lets your body adjust to the new
medicine. The goal of titration is to reach the highest dose you can tolerate. This
can be up to a maximum dose of 1600 micrograms twice a day.
During titration, you will receive a titration pack containing light yellow UPTRAVI
200 micrograms tablets. These tablets have a “2” stamped on one side. Your doctor
will tell you to increase your dose in steps, usually every week. With each step,
you will add one 200 micrograms tablet to your morning and evening doses. The first
intake of the increased dose should be in the evening. Below is a diagram to show
the dose and the number of tablets at each step for the first 4 steps.
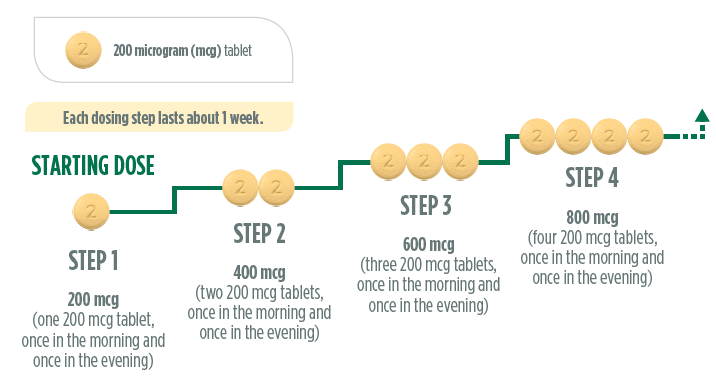
If you reach a dose of 800 micrograms in the morning and 800 micrograms in the evening,
your doctor may instruct you to increase your dose to 1000 micrograms in the morning
and 1000 micrograms in the evening. This will be done by taking one green 800 micrograms
tablet and adding one light-yellow 200 micrograms tablet. For this, you will receive
a pack containing green UPTRAVI 800 micrograms tablets (with an “8” stamped on one
side) and a titration pack containing light yellow UPTRAVI 200 micrograms tablets
(with a “2” stamped on one side).
Your doctor will tell you to increase your dose in steps, usually every week. As before,
with each step, you will add a 200 micrograms tablet to your morning and evening doses.
The first intake of the increased dose should be in the evening. The maximum dose
of UPTRAVI is 1600 micrograms in the morning and in the evening. However, not every
patient will reach this dose.
Below is a diagram to show the dose and the number of tablets at each step starting
with step 5.
You will receive a titration guide providing information on how to increase your dose
and allowing you to record the number of tablets you take every day. Remember to record
the number of tablets you take every day. Remember to talk to your PAH doctor or nurse
regularly during titration.
Stepping down to a lower dose due to side effects
During titration, you may experience side effects such as headache, jaw pain, aching
joints, muscle pain or a general feeling of being in pain, diarrhoea, feeling sick,
being sick or reddening of the face. If these side effects are difficult for you to
tolerate, talk to your doctor about how to manage or treat them. There are treatments
available that can help relieve the side effects. Do not stop taking UPTRAVI unless
your doctor tells you to.
If the side effects cannot be treated or do not gradually get better on the dose you
are taking, your doctor may adjust your dose by reducing by one the number of 200
micrograms yellow tablets you take in the morning and in the evening. The diagram
below shows stepping down to a lower dose. Do this only if instructed to do so by
your doctor.
Do not stop taking UPTRAVI unless your doctor tells you to.
If your side effects are manageable after stepping down your dose, your doctor may
decide that you should continue to stay at that level. Please see section "Maintenance
dose" below for more information.
Maintenance dose
The highest dose that you can tolerate during titration will become your maintenance
dose. Your maintenance dose is the dose you should continue to take on a regular basis,
in the morning and in the evening with the minimum side effects.
Every patient with PAH is different. Not everyone will end up on the same maintenance
dose. Your maintenance dose will be between 200 micrograms and 1600 micrograms in
the morning and in the evening. What is important is that you reach the dose that
is most appropriate to treat you.
Your doctor can prescribe an equivalent single-tablet strength for your maintenance
dose.
This allows you to take one tablet in the morning and one in the evening, instead
of multiple tablets for each.
The single tablet will be of a different colour depending on the dose. Each tablet
will have a number on its surface showing the dose (in hundreds of micrograms). For
a full description including colours and marking, please see Product details section
of this leaflet.
Over time, your doctor may adjust your maintenance dose as needed.
If, at any time, after taking the same dose for a long time, you experience side effects
that you cannot tolerate or side effects that have an impact on your normal daily
activities, contact your doctor.
Do not stop taking UPTRAVI unless your doctor tells you to.
When to take UPTRAVI
Take UPTRAVI in the morning and in the evening, consistently either with or without
meals. You might tolerate the medicine better when you take it with meals.
How to take UPTRAVI
Swallow the tablets whole, do not split, crush or chew the UPTRAVI tablets.
If you forget to use UPTRAVI
UPTRAVI should be used regularly at the same time each day. If you miss your dose
at the usual time, take a dose as soon as you remember, then continue to take your
tablets at the usual times.
If it is almost time for your next dose (within six hours before you would normally
take it), skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
Do not stop taking UPTRAVI unless your doctor tells you to. If, for any reason, you
stop taking UPTRAVI for more than 3 consecutive days (if you missed 3 morning and
3 evening doses or 6 doses in a row or more), contact your doctor immediately as your
dose may need to be adjusted to avoid side effects. Your doctor may decide to restart
your treatment on a lower dose, gradually increasing to your previous maintenance
dose.
If you use too much UPTRAVI
If you think that you have used too much UPTRAVI, you may need urgent medical attention.
You should immediately:
phone the Poisons Information Centre (in Australia telephone 13 11 26 and in New Zealand telephone 0800 POISON or 0800 764 766), or
contact your doctor, or
go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using UPTRAVI?
Things you should do
Tell your doctor or pharmacist that you are taking UPTRAVI if you are about to start
on any new medicines.
Tell your doctor if you become pregnant or are trying to become pregnant.
Tell your doctor if you are breastfeeding or plan to breastfeed.
Tell your doctor if, for any reason, you have not used your medicine exactly as prescribed.
Keep all of your doctor or clinic appointments.
Your doctor may do certain tests, including blood tests, from time to time to make
sure the medicine is working and to prevent unwanted side effects.
Remind any doctor or pharmacist you visit that you are taking UPTRAVI.
Things you should not do
Do not stop using this medicine unless your doctor tells you to.
Do not give this medicine to anyone else, even if their symptoms seem similar to yours.
Do not use UPTRAVI to treat any other complaints unless your doctor says to.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how UPTRAVI
affects you.
UPTRAVI can cause side effects such as headaches and low blood pressure and the symptoms
of your condition can also make you less fit to drive. If you are affected, do not
drive or operate machinery.
Looking after your medicine
Store below 30°C. Protect from moisture.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do
not store it:
in the bathroom or near a sink, or
in the car or on window sills.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy
for safe disposal.
Do not use this medicine after the expiry date. The expiry date refers to the last
day of that month.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of
them are minor and temporary. However, some side effects may need medical attention.
You may experience side effects not only during the titration period when your dose
is being increased, but also later after taking the same dose for a long time.
If you experience any of these side effects: headache, jaw pain, aching joints, muscle
pain or a general feeling of being in pain, diarrhoea, feeling sick, being sick or
reddening of the face, that you cannot tolerate or that cannot be treated, you should
contact your doctor as the dose you are taking may be too high for you and may need
to be reduced.
See the information below and, if you need to, ask your doctor or pharmacist if you
have any further questions about side effects.
Less serious side effects
Serious side effects
|
Serious side effects
|
What to do
|
|
Signs of an allergic reaction such as:
wheezing, shortness of breath, difficulty breathing, or a tight feeling in your chest
swelling of the face, lips, tongue or other parts of the body
rash, itching, hives or flushed red skin
dizziness or light-headedness.
Signs of kidney failure such as:
decreased urine output
swelling in the ankles or feet
tiredness or weakness
shortness of breath
confusion
irregular heartbeat
|
Call your doctor straight away, or go straight to the Emergency Department at your
nearest hospital if you notice any of these serious side effects.
|
Tell your doctor or pharmacist if you notice anything else that may be making you
feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
Always make sure you speak to your doctor or pharmacist before you decide to stop
taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What UPTRAVI contains
|
Active ingredient
(main ingredient)
|
selexipag
|
|
Other ingredients
(inactive ingredients)
|
Tablet core:
mannitol, maize starch, hyprolose, magnesium stearate
Film coat:
200 micrograms:
hypromellose, propylene glycol, titanium dioxide, iron oxide yellow, carnauba wax.
400 micrograms:
hypromellose, propylene glycol, titanium dioxide, iron oxide red, carnauba wax.
600 micrograms:
hypromellose, propylene glycol, titanium dioxide, iron oxide red, iron oxide black,
carnauba wax.
800 micrograms:
hypromellose, propylene glycol, titanium dioxide, iron oxide yellow, iron oxide black,
carnauba wax.
1000 micrograms:
hypromellose, propylene glycol, titanium dioxide, iron oxide red, iron oxide yellow,
carnauba wax.
1200 micrograms:
hypromellose, propylene glycol, titanium dioxide, iron oxide black, iron oxide red,
carnauba wax.
1400 micrograms:
hypromellose, propylene glycol, titanium dioxide, iron oxide yellow, carnauba wax.
1600 micrograms:
hypromellose, propylene glycol, titanium dioxide, iron oxide black, iron oxide red,
iron oxide yellow, carnauba wax.
|
|
Potential allergens
|
n/a
|
Do not take this medicine if you are allergic to any of these ingredients.
What UPTRAVI looks like
UPTRAVI 200 micrograms film-coated tablets are: Round, light yellow, film-coated tablets
with “2” marked on one side. (AUST R 234161).
UPTRAVI 400 micrograms film-coated tablets are: Round, red, film-coated tablets with
“4” marked on one side. (AUST R 234160)
UPTRAVI 600 micrograms film-coated tablets are: Round, light violet, film-coated tablets
with “6” marked on one side. (AUST R 234159)
UPTRAVI 800 micrograms film-coated tablets are: Round, green, film-coated tablets
with “8” marked on one side. (AUST R 234166)
UPTRAVI 1000 micrograms film-coated tablets are: Round, orange, film-coated tablets
with “10” marked on one side. (AUST R 234162)
UPTRAVI 1200 micrograms film-coated tablets are: Round, dark violet, film-coated tablets
with “12” marked on one side. (AUST R 234163)
UPTRAVI 1400 micrograms film-coated tablets are: Round, dark yellow, film-coated tablets
with “14” marked on one side. (AUST R 234165)
UPTRAVI 1600 micrograms film-coated tablets are: Round, brown, film-coated tablets
with “16” marked on one side. (AUST R 234164)
Who distributes UPTRAVI
JANSSEN-CILAG Pty Ltd
1-5 Khartoum Road
Macquarie Park NSW 2113 Australia
Telephone: 1800 226 334
NZ Office: Auckland New Zealand
Telephone: 0800 800 806
This leaflet was prepared on 13 January 2025.