This article is based on a poster originally authored by Luiza Chrojan, Lauren Thomlinson, Kirsty McManus, Charlotte Hands, Patrick Hoering, James Brown, Sophie Thompson, Rebecca Rich, Lucy Ballantine, Michael Walker and John Allen.
Introduction
Pharmaron Biologics, located in Liverpool, UK, is a commercial development and manufacturing organization (CDMO) that provides a range of end-to-end laboratory services for the development and production of complex biological therapies, including monoclonal antibodies (mAbs).
To ensure the integrity of these biologics, Critical Quality Attributes (CQAs) must be closely monitored throughout the production process.
A case study of forced degradation is presented using two commercially available IgG1 monoclonal antibodies (mAbs). Advanced analytical technology has been used, such as:

Image Credit: Pharmaron
Pharmaron offers comprehensive CQA profiling with high sensitivity and throughput, thereby reducing analytical costs and expediting timelines.
These findings demonstrate Pharmaron's capacity to enable robust biologics development and validation using unique, high-resolution analytical methodologies.
Pharmaron’s analytical capabilities
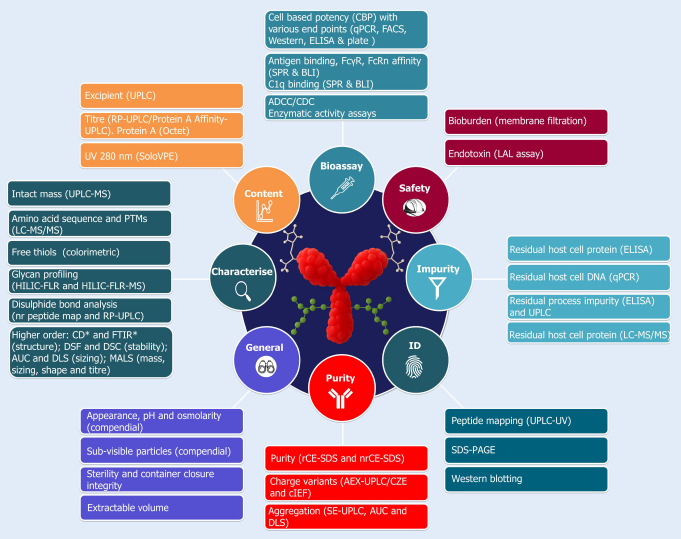
Image Credit: Pharmaron
Methods & results summary
In this investigation, two separate batches of commercially available IgG1 (A and B) were subjected to degradation using high/low pH, oxidative stress, and elevated temperatures to assess their stability patterns.
Table 1. Forced Degraded Results Summary. Source: Pharmaron
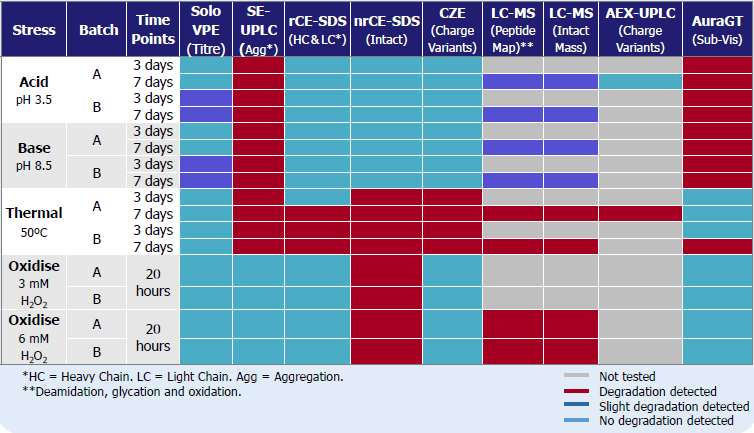
Results
SE-UPLC & SEC-MALS
- Batches A and B were separated using size exclusion ultra-performance liquid chromatography (SE-UPLC) using an Acquity Premier UPLC and column. Both demonstrated a reduction in purity after acid, base, and 50 °C heat treatment (Figure A).
- Both batches exhibited significant aggregation after seven days at 50 °C (see Figure B). Figure C depicts the aggregate species of batch A after seven days at 50 °C with size exclusion-MALS (SEC-MALS) on Dawn. These are composed of bigger aggregates.
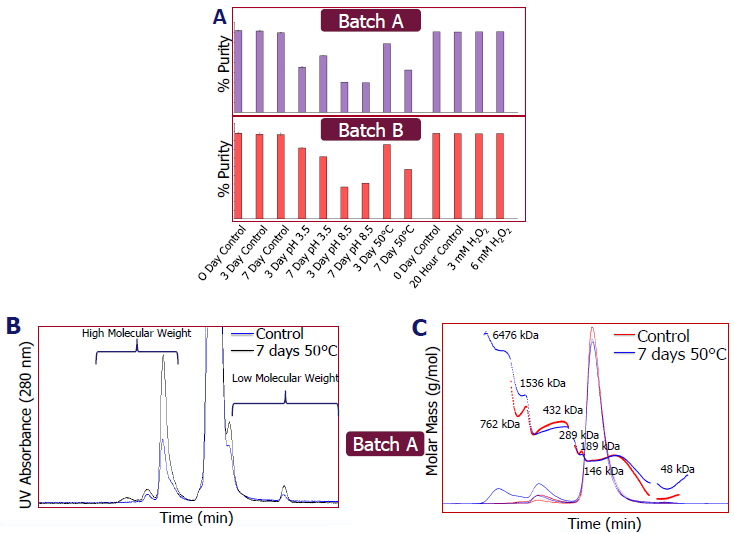
Image Credit: Pharmaron
CZE & CE-SDS
- The SCIEX CESI 8000 Plus used Capillary Zone Electrophoresis (CZE) to separate charge variants. Both batches had the greatest rise in charge variations after seven days at 50 °C. Figure D shows a representative batch.
- The Biophase 8800 measured LC, HC, and glycans using reduced CE (rCE)-SDS. Again, 50 °C for seven days caused the most noticeable modifications in both batches, as illustrated in Figure E for one batch (dotted line represents mAb fragments).
- To check intact monomer and fragmentation, nonreduced-CE (nrCE)-SDS was used. 50 °C for seven days resulted in the greatest loss in purity (Figure F).
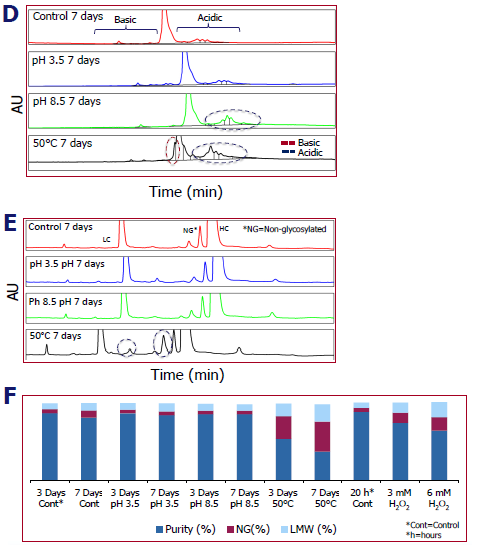
Image Credit: Pharmaron
Intact mass & peptide map
- Samples were separated using reversed-phase UPLC (RP-UPLC) before measuring total molecular weight using the Vion MS instrument. This demonstrated that the batch masses were equivalent (Figure G).
- mAbs were digested with trypsin and separated using RP-UPLC-MS on the Lumos MS. Both batches exhibited oxidation on M258 and M364 following H2O2 treatment (see Figure H).
- In Batch A, oxidation of C194 and W108 occurred after seven days at 50 °C (Figure H).
- For both batches, heat and acid treatment resulted in hot spot deamidation at N321 and N331 (see Figure I).
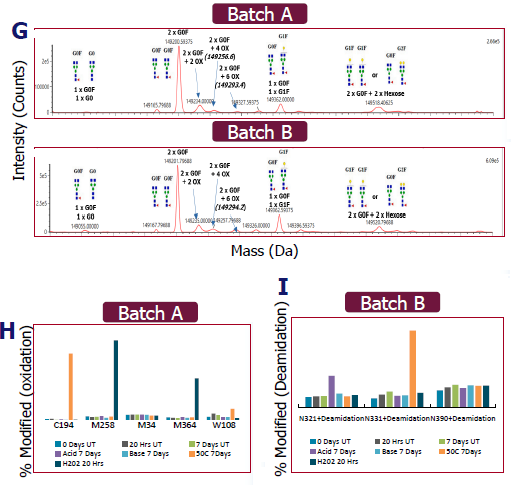
Image Credit: Pharmaron
Conclusions
Pharmaron's novel and highly sensitive platform approaches enable a comprehensive investigation of various mAb CQAs.
These in-house methods are easily available to assist with the process characterization, comparability, and validation of early to late-stage products.
Using fit-for-purpose data streamlines the path to clinical application, resulting in faster access to potentially life-changing treatments.
About Pharmaron
Pharmaron (Stock Code: 300759.SZ/3759.HK) is a premier R&D service provider for the life sciences industry. Founded in 2004, Pharmaron has invested in its people and facilities, and established a broad spectrum of research, development, and manufacturing service capabilities throughout the entire drug discovery, preclinical, and clinical development process across multiple therapeutic modalities, including small molecules, biologics, and CGT products. With over 17,000 employees and operations in China, the U.S., and the U.K., Pharmaron has an excellent track record in the delivery of R&D solutions to its partners in North America, Europe, Japan, and China.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.net, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.
Last Updated: Jan 9, 2026