This article and associated images are based on a poster originally authored by Sarona Jacob Anderson and presented at ELRIG Drug Discovery 2025 in affiliation with University of Salford.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Abstract
The p53–MDM2 regulatory pathway is crucial for genomic integrity, with its dysregulation contributing to over half of all cancers.
MDM2 overexpression suppresses p53's tumor-suppressive activity by promoting its degradation. This study aimed to identify natural product inhibitors of the p53–MDM2 interaction using computational methods.
Phytochemicals from traditional medicinal plants with anticancer potential were screened using BIOVIA Discovery Studio. Lipinski and ADMET filters prioritized compounds with favourable drug-like properties.
Molecular docking (CDOCKER, LibDock) identified potential binders to MDM2 (PDB: 1YCR). Molecular dynamics simulations assessed binding stability (RMSD/RMSF).
Toxicity was predicted using TopKat. Binding energies were compared with clinical MDM2 inhibitors.
Octanamide, n-(2- mercaptoethyl)- emerged as the top candidate, showing strong binding affinity, excellent dynamic stability, favorable predicted safety, and comparable binding energy to several clinical candidates.
This study highlights Octanamide, n-(2-mercaptoethyl)- as a promising natural scaffold for developing safer p53-reactivating cancer therapeutics.
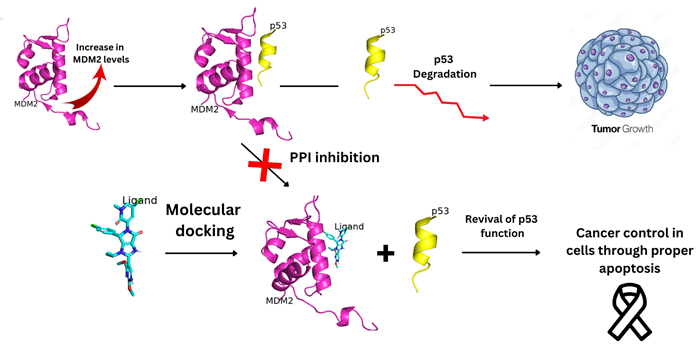
Fig 1. Graphical Overview of p53-MDM2 interaction. Image Credit: Image courtesy of Sarona Jacob Anderson et al., in partnership with ELRIG (UK) Ltd.
Introduction
Cancer remains a leading cause of mortality, often linked to the failure of apoptosis, or programmed cell death, which normally eliminates damaged cells.
The p53 protein, known as the "guardian of the genome," plays a vital role by inducing cell cycle arrest or apoptosis in response to cellular stress, thereby maintaining genomic integrity.
Dysfunction in the p53 pathway contributes to over 50 % of human cancers.
MDM2 acts as a key negative regulator, binding to p53's N-terminal domain to inhibit its activity and target it for ubiquitin-mediated degradation.
Overexpression or amplification of MDM2 silences p53, promoting tumor progression. Therefore, inhibiting the p53-MDM2 interaction represents an effective therapeutic strategy to reactivate p53.
While synthetic small-molecule MDM2 inhibitors, such as Nutlins, have shown efficacy, their clinical utility is often limited by issues including toxicity and the development of resistance.
Consequently, natural products, particularly phytochemicals from medicinal plants, are being explored as alternative scaffolds due to their vast structural diversity and potentially favorable safety profiles.
Integrating traditional knowledge of medicinal plants with modern computational drug discovery techniques offers a powerful approach to accelerate the identification of novel therapeutic agents.
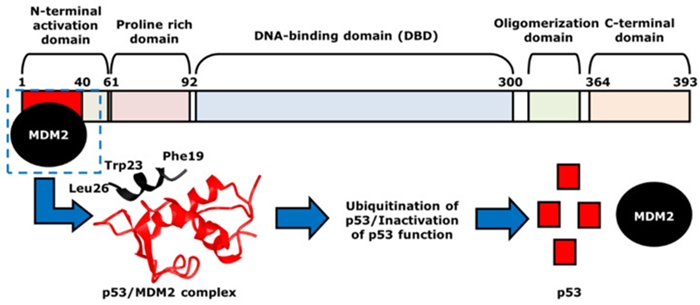
Fig 2. The mechanism of p53- and MDM2-binding. MDM2, indicated as black, binds to p53 at the N-terminal activation domain of p53, or the red area, to form p53/MDM2 complex. Image Credit: Koo et al., 2022
Methodology
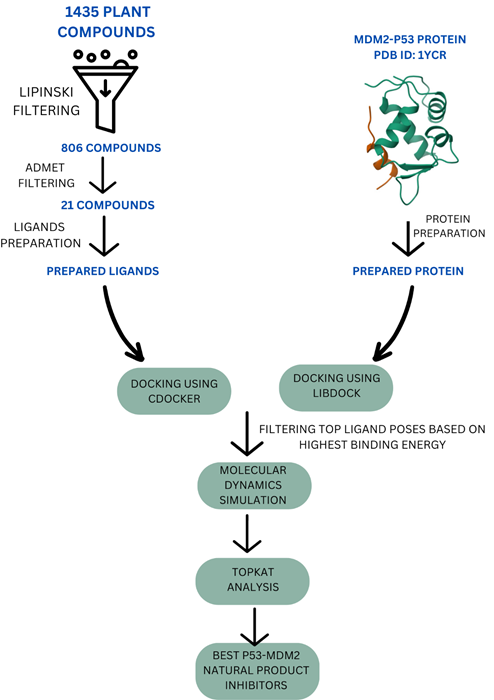
Fig 3. Methodology of the study. Image Credit: Image courtesy of Sarona Jacob Anderson et al., in partnership with ELRIG (UK) Ltd.
Results
- Filtering: 21 natural compounds passed Lipinski and stringent ADMET filters, showing favorable drug-like properties. (Figure 4 )
- Docking: CDOCKER: Octanamide, n-(2-mercaptoethyl)- ranked 2nd with -30.51 kcal/mol. Top 5 included Phenethylamine, alpha-ethyl-, N-(4-hydroxyundecanoyl)anabasine, Butanoic acid, butyl ester, and Methyl heptanoate.
- LibDock: Octanamide, n-(2-mercaptoethyl)- ranked 4th with a score of 77.27. Top 5 included N-(4-hydroxyundecanoyl) anabasine, N-n-octanoylnornicotine, Capsaicin, and 2-cyclohexylpiperidine .
- Octanamide, n-(2-mercaptoethyl)- showed key interactions including hydrogen bonds (TRP B:23, GLU B:28, LYS B:24) and hydrophobic contacts within the p53-binding pocket. ( Figure 5)
- Binding Energy: Octanamide, n-(2-mercaptoethyl)- (-2.36 kcal/mol) showed better calculated binding energy than Siremadlin (-2.29), Navtemadlin (-2.12), SAR405838 (-1.98), and Nutlin-3A (-0.22 kcal/mol)
- MD Stability (RMSD): Octanamide, n-(2-mercaptoethyl)- displayed the lowest average RMSD (0.94 Å) among the top 5 compounds, indicating high structural stability of the complex during the simulation. (Figure 6).
- Residue Flexibility (RMSF): The Octanamide-MDM2 complex showed low fluctuations in key binding site residues (e.g., ILE61: 0.445 Å, THR63: 0.434 Å), suggesting stable interactions. (Figure 7).
- Toxicity Prediction (TopKat): Octanamide, n-(2-mercaptoethyl)- was predicted to be non-mutagenic and non-carcinogenic in mouse and rat models.
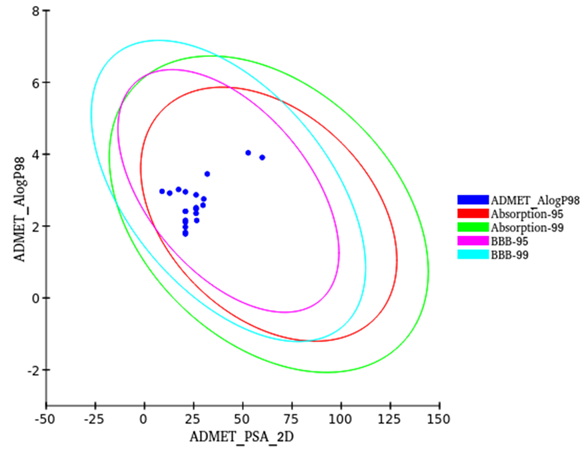
Fig 4. ALogP versus polar surface area (PSA) plot for compounds showing the 95 % and 99 % confidence. Image Credit: Image courtesy of Sarona Jacob Anderson et al., in partnership with ELRIG (UK) Ltd.
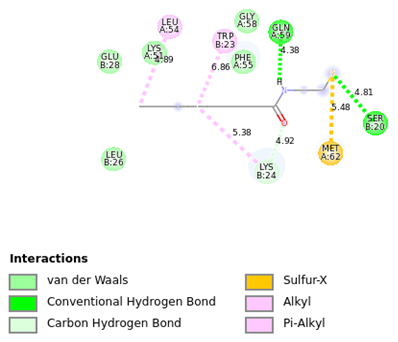
Fig 5. 2D diagram of Octanamide in 1YCR at the binding site. Image Credit: Image courtesy of Sarona Jacob Anderson et al., in partnership with ELRIG (UK) Ltd.
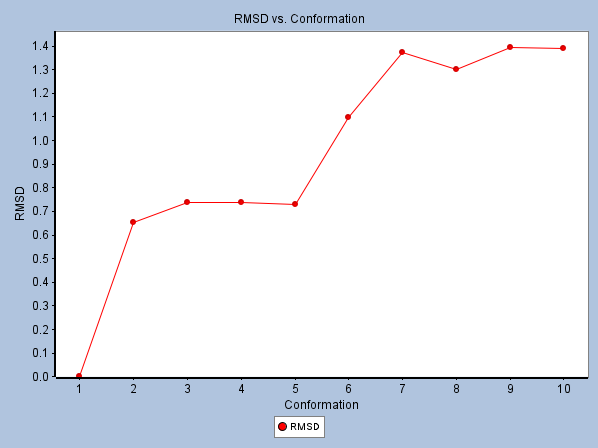
Fig 6. RMSD graph of Octanamide, n-(2-mercaptoethyl)- with 1YCR. Image Credit: Image courtesy of Sarona Jacob Anderson et al., in partnership with ELRIG (UK) Ltd.
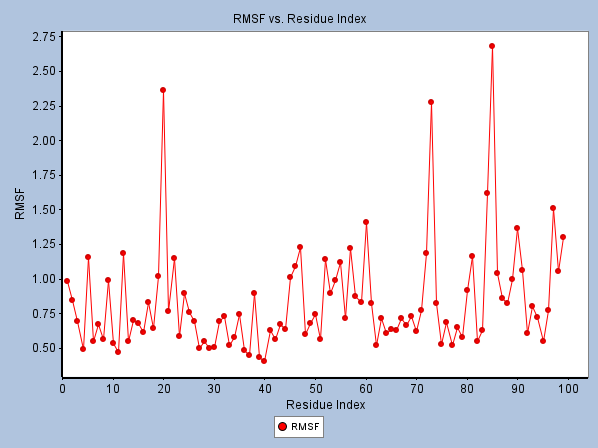
Fig 7. RMSF Analysis of Octanamide, N-(2-Mercaptoethyl)-1YCR Complex. Image Credit: Image courtesy of Sarona Jacob Anderson et al., in partnership with ELRIG (UK) Ltd.
Discussion
Targeting the p53-MDM2 interaction remains a key strategy in oncology, but clinical translation of synthetic inhibitors is challenged by toxicity and limited efficacy
Natural products offer a vast chemical diversity for identifying novel scaffolds. Previous studies identified compounds like Lineariifolianoid A and lignans as potential MDM2 inhibitors
This study employed a rigorous computational approach, combining filtering, docking, MD simulations, and toxicity prediction to screen phytochemicals from anticancer plants
Octanamide, n-(2-mercaptoethyl)-, identified from Vitis vinifera, emerged as the top candidate. It demonstrated:
- Strong predicted binding affinity (high ranks in CDOCKER and LibDock)
- Favourable calculated binding energy compared to several clinical candidates
- High stability in the MDM2 binding pocket (lowest RMSD)
- Indication of stable binding interactions (low RMSF in key residues)
- Predicted non-mutagenic and non-carcinogenic profile
These findings highlight the potential of computationally screened natural products as starting points for developing safer and effective p53-reactivating therapies.
Conclusion
This computational investigation successfully employed a multi-faceted in silico workflow to identify promising natural product-based inhibitors targeting the p53-MDM2 interaction.
Among the evaluated phytochemicals, Octanamide, n-(2-mercaptoethyl)- from Vitis vinifera distinguished itself through strong predicted binding affinity, excellent stability in the MDM2 binding pocket confirmed by MD simulations, and a favorable predicted safety profile.
These findings strongly support its potential as a lead candidate for restoring p53 function.
This study highlights the importance of combining traditional plant-based medicine knowledge with modern computational chemistry to identify and prioritize natural compounds, such as Octanamide, n-(2-mercaptoethyl)-, for the development of safer anticancer therapeutics. Further experimental validation and structure-based optimization are warranted to advance this promising natural scaffold.
References
- Kussie, P.H., et al. (1996). Structure of the MDM2 Oncoprotein Bound to the p53 Tumor Suppressor Transactivation Domain. Science, 274(5289), pp.948–953. https://doi.org/10.1126/science.274.5289.948.
- Koo, N., Sharma, A.K. and Narayan, S. (2022). Therapeutics Targeting p53-MDM2 Interaction to Induce Cancer Cell Death. International Journal of Molecular Sciences, 23(9), p.5005. https://doi.org/10.3390/ijms23095005.
About the University of Salford
The University of Salford is a forward-thinking institution located in Greater Manchester, known for its strong industry connections and commitment to practical, career-focused learning. Established in 1896 as the Royal Technical Institute, Salford has evolved into a dynamic university with a global reputation for excellence in teaching, research, and innovation.
Salford’s research is internationally r
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free to attend!
Our values
Our values are to always ensure the highest quality of content, that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer-led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world-class conferences, networking events, webinars, and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Nov 27, 2025