This article and associated images are based on a poster originally authored by Lalan Kumar, Nico Boback, Badri Parshad, Marlena Schlecht, Thorsten Wolff, Anil Kumar Sahoo, Andreas Hocke, Daniela Niemeyer, Daniel Lauster and Sumati Bhatia and presented at ELRIG Drug Discovery 2025 in affiliation with Swansea University, Institute for Chemistry and Biochemistry, Harvard Medical School, Robert Koch Institute, Charité-Universitätsmedizin Berlin and Freie Universität Berlin.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Heteromultivalent dual action glycosystems against influenza A virus infections
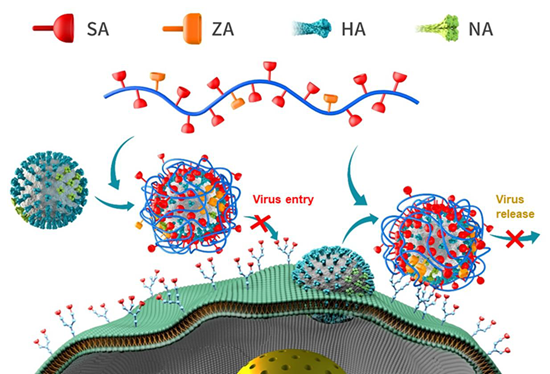
Binding and release inhibition. (SA = Sialic acid, ZA = Zanamivir, HA = Hemagglutinin, NA = Neuraminidase). Image Credit: Image courtesy of Lalan Kumar et al., in partnership with ELRIG (UK) Ltd.
Here, we demonstrate the concerted inhibition of different IAV strains using a low-molecularweight dual-action linear polymer. Polyglycerol-based scaffolds stand out due to their excellent water solubility, biocompatibility, and chemical versatility, making them particularly suitable for a wide range of biological applications - from drug delivery to bioengineering. These polymers can be readily modified with various functional units, enabling targeted interactions with specific molecules or surfaces. A major advancement in the field of antiviral polymers is the development of linear polyglycerol (LPG)-based heteromultivalent polymers functionalized with both 6′-sialyllactose (SA) and zanamivir (ZA). These polymers are engineered to simultaneously target two key surface proteins of the influenza A virus (IAV): hemagglutinin (HA), which mediates viral attachment to host cells, and neuraminidase (NA), which facilitates the release of new viral particles. Conjugation of these two ligands on a single polymer chain yields heteromultivalent polymers with potent antiviral activity via a dual-action mechanism. The 6′-sialyllactose moiety mimics natural sialic acid receptors, enhancing HA binding, while zanamivir inhibits NA activity, thereby blocking viral replication. Regardless of IAV subtype, binding inhibition studies indicate that heteromultivalent polymers exhibit superior virus adsorption compared to their homomultivalent counterparts. Our results demonstrate that the optimized presentation of ZA and SA on the PG backbone can efficiently inhibit the propagation of various IAV strains at very low nanomolar concentrations in vitro, achieving >99.9 % infection inhibition - approximately 10,000 times more effective than the commercial ZA drug. Building on this strategy, multivalent sialosides have also shown 500-fold stronger binding affinity against SARS-CoV-2 than highly sulfated analogs, underscoring the importance of leveraging sialoglycan-based designs for antiviral applications.
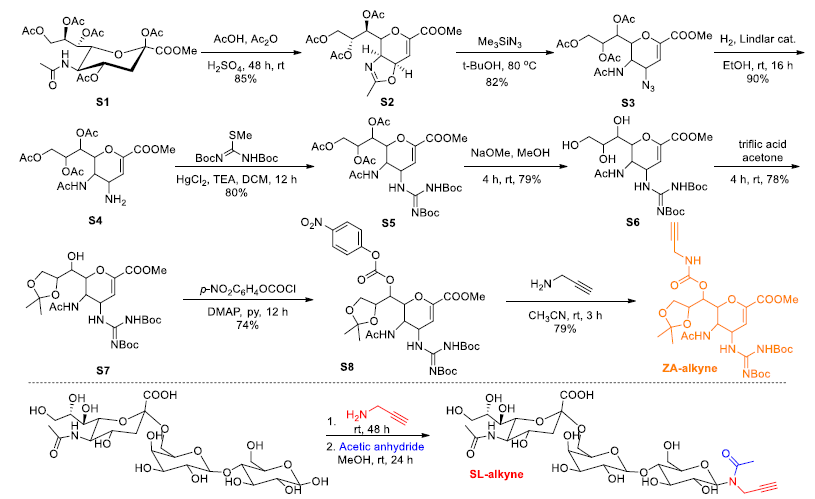
Synthesis of ZA-alkyne and SL-alkyne. Image Credit: Image courtesy of Lalan Kumar et al., in partnership with ELRIG (UK) Ltd.
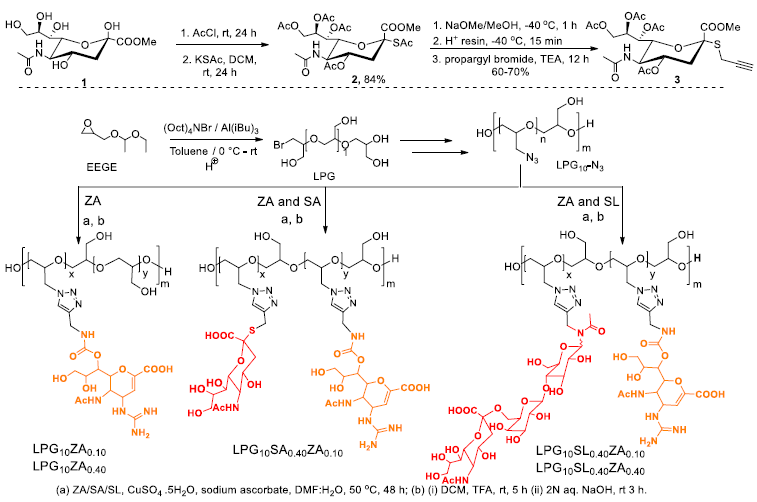
Synthesis of SA-alkyne and LPG. Image Credit: Image courtesy of Lalan Kumar et al., in partnership with ELRIG (UK) Ltd.
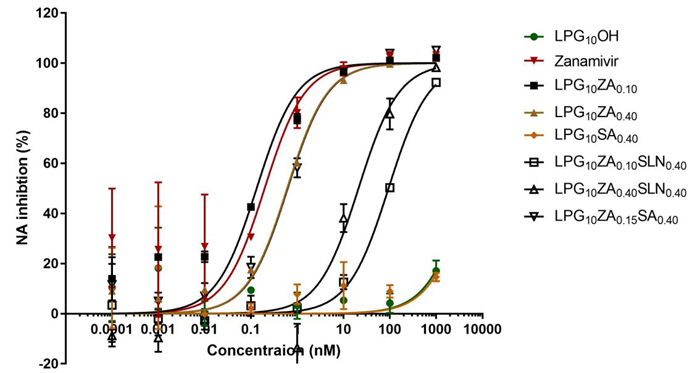
NA inhibition. Image Credit: Image courtesy of Lalan Kumar et al., in partnership with ELRIG (UK) Ltd.
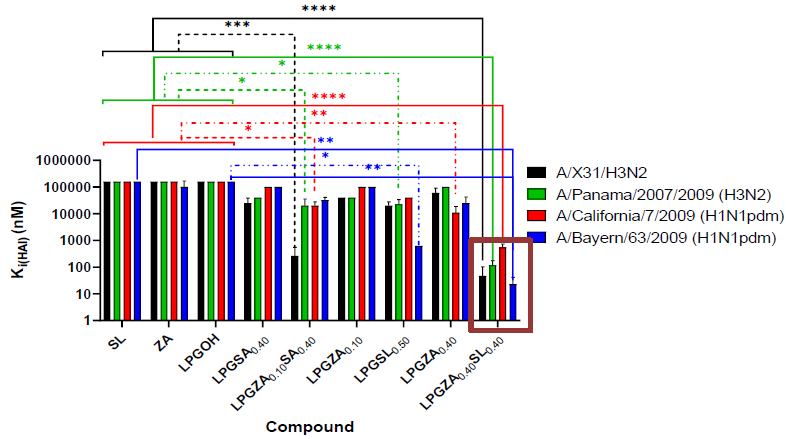
Hemagglutinnation inhibition. Image Credit: Image courtesy of Lalan Kumar et al., in partnership with ELRIG (UK) Ltd.
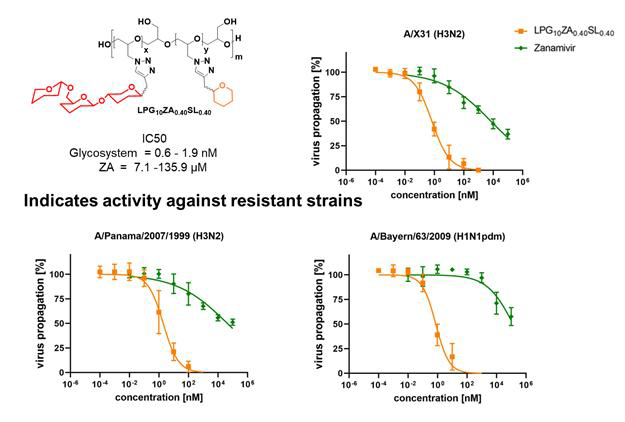
Infection inhibition. Image Credit: Image courtesy of Lalan Kumar et al., in partnership with ELRIG (UK) Ltd.
Polysialosides vs. Polysulphates: Synthesis and inhibition of SARS-CoV-2
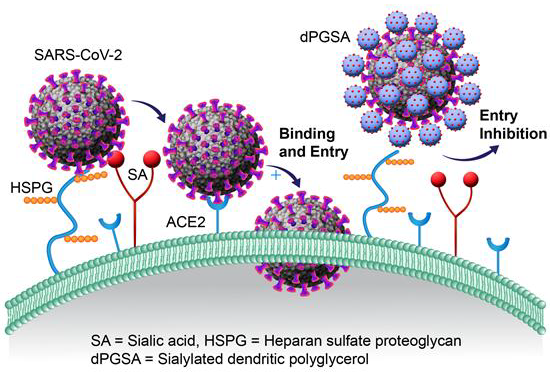
Image Credit: Image courtesy of Lalan Kumar et al., in partnership with ELRIG (UK) Ltd.
Both polysialosides and polysulfates are known to interact with the receptor binding domain (RBD) of the SARS-CoV-2 spike protein. However, a comprehensive site by site analysis of their binding affinities and potential synergistic antiviral effects have not been performed. Here, we report on the synthesis of polysialosides with nanomolar binding affinities to spike proteins of SARS-CoV-2 in solution using microscale thermophoresis. The dendritic polyglycerol based polysialosides bind to SARS-CoV-2 at low nM concentrations which is ~500 times stronger than the high density polysulfated analog. In fact, the presence of sulfate groups in a heteromultivalent compound weakens the binding to spike proteins. A polycarboxylated analog does not bind to SARS-CoV-2, ruling out that the interaction of polysialoside is simply driven by electrostatics. Explicit-solvent all-atom molecular dynamics simulations and ensemble docking studies support the conclusion that sialosides interact stronger than sulfates for their binding with RBD of SARS-CoV-2. Notably, our most affine binder inhibits SARS-CoV-2 (WT, D614G) replication up to 98.6 % at 0.5 μM.
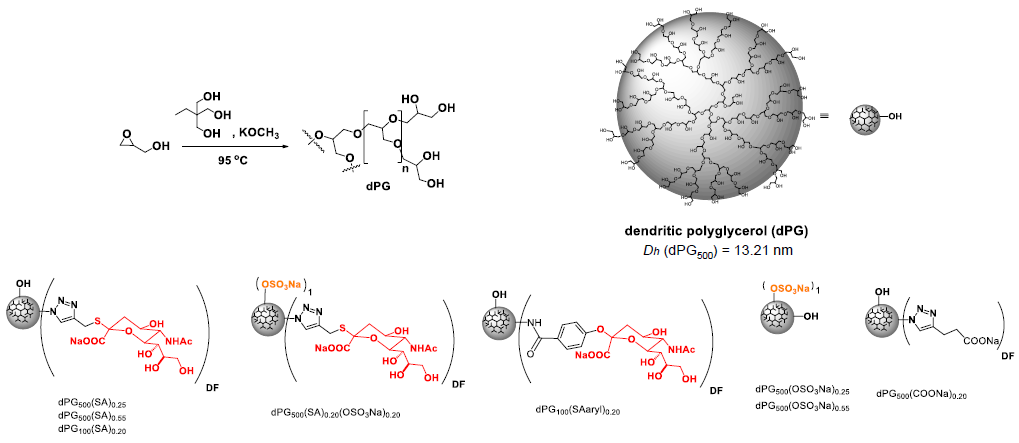
Image Credit: Image courtesy of Lalan Kumar et al., in partnership with ELRIG (UK) Ltd.
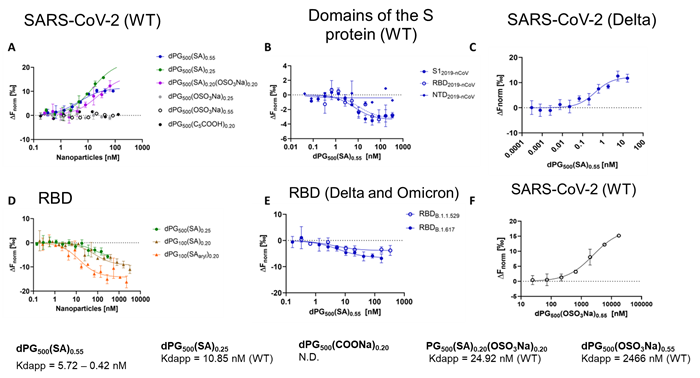
Binding affinity analysis using microscale thermophoresis (MST). Image Credit: Image courtesy of Lalan Kumar et al., in partnership with ELRIG (UK) Ltd.
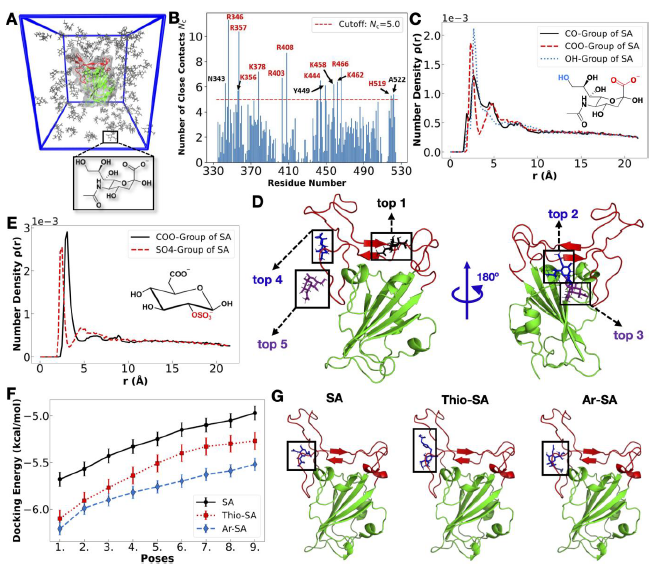
Explicit-solvent atomistic MD simulation and molecular docking studies. Image Credit: Image courtesy of Lalan Kumar et al., in partnership with ELRIG (UK) Ltd.
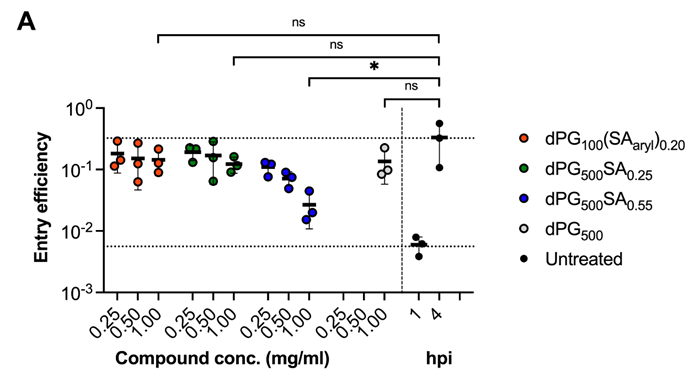
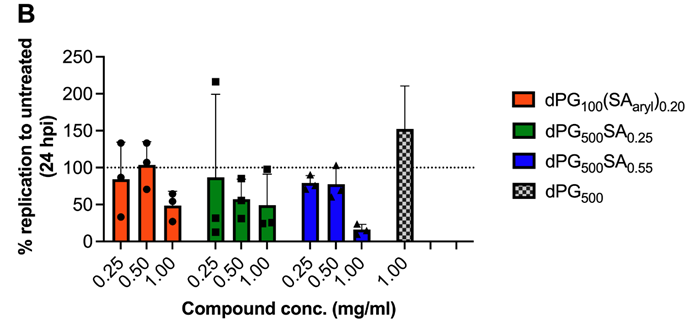
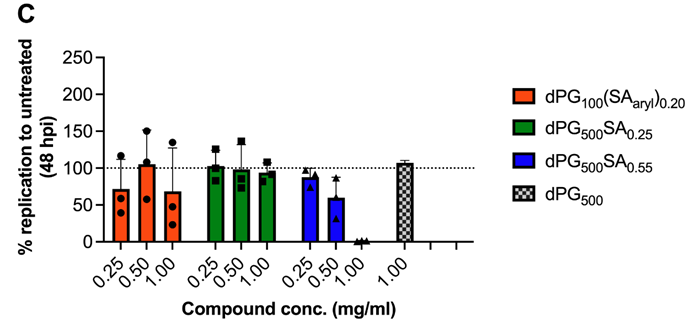
Entry inhibition (A) and replication inhibition (B,C). Image Credit: Image courtesy of Lalan Kumar et al., in partnership with ELRIG (UK) Ltd.
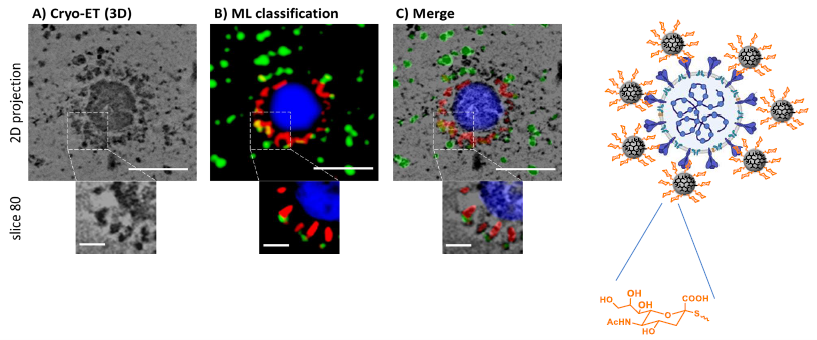
Explicit-solvent atomistic MD simulation and molecular docking. Image Credit: Image courtesy of Lalan Kumar et al., in partnership with ELRIG (UK) Ltd.
Conclusions
The development of heteromultivalent polyglycerol-based polymers functionalized with both 6′-sialyllactose and zanamivir represents a significant advancement in antiviral polymer design. By targeting both hemagglutinin and neuraminidase on the influenza A virus, these dual-action polymers exhibit remarkable antiviral efficacy, achieving
>99.9 % inhibition at nanomolar concentrations-vastly outperforming conventional monotherapies. The success of this strategy highlights the critical role of multivalency and precise molecular architecture in enhancing virus-binding affinity and blocking replication. Furthermore, the promising application of sialoglycan-based multivalents
against SARS-CoV-2 suggests a broad potential for this platform in developing next-generation antiviral agents.
References
- Badri Parshad, et al. (2023). Dual-Action Heteromultivalent Glycopolymers Stringently Block and Arrest Influenza A Virus Infection In Vitro and Ex Vivo. Nano letters, 23(11), pp.4844–4853. https://doi.org/10.1021/acs.nanolett.3c00408.
- Khatri, V., et al. (2025). Polysialosides Outperform Sulfated Analogs for Binding with SARS-CoV-2. PubMed, pp.e2500719–e2500719. https://doi.org/10.1002/smll.202500719.
- Cuellar-Camacho, J.L., et al. (2020). Quantification of Multivalent Interactions between Sialic Acid and Influenza A Virus Spike Proteins by Single-Molecule Force Spectroscopy. Journal of the American Chemical Society, 142(28), pp.12181–12192. https://doi.org/10.1021/jacs.0c02852.
- Stadtmueller, M.N., et al. (2021). Evaluation of Multivalent Sialylated Polyglycerols for Resistance Induction in and Broad Antiviral Activity against Influenza A Viruses. Journal of Medicinal Chemistry, 64(17), pp.12774–12789. https://doi.org/10.1021/acs.jmedchem.1c00794.
About Swansea University
Since 1920, Swansea University has been producing world-class research with a strong tradition of collaboration with business and industry. Today, its research extends far beyond these partnerships, influencing health, wealth, culture, and the well-being of societies across the globe.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free-of-charge to attend!
Our values
Our values are to always ensure the highest quality of content and that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world class conferences, networking events, webinars and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Nov 27, 2025