This article and associated images are based on a poster originally authored by Adedapo Adesokan and Abubakar Abdulhakim and presented at ELRIG Drug Discovery 2025 in affiliation with Precisemed Limited and Ahmadu Bello University.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Introduction
- The roadmap for R&D of novel pharmaceutical therapies typically involves conceptualization, formulation, and preclinical and human trial tests.
- For the Dementia Support Medicinal product, the researchers selected the Oral Thin Film Strips (OTF) delivery system for its efficiency.
- Considering the unmet clinical needs in dementia therapeutics, where available drugs lack efficacy, Dementia Support OTF, intended as a Class 2A medical device once licensed by the FDA and MHRA, is being developed with a 1st-in-human Dementia Support Probiotic.
Novel dementia support OTF:
- The development of OTF is divided into three phases:
-
-
- Stage 1: Preclinical Subacute toxicity
- Stage 2: Behavioural and Biomarkers efficacy
- Stage 3: Human Trial from Phase 1 to Phase 3/4 to demonstrate OTF and Probiotics are not toxic, but safe and efficacious
- The first two phases of the study are now complete and are described here, while the latter phase is pending.
1st -in -human dementia support probiotics:
Hypothesis underlying the development of the first In-Human Dementia Support Probiotics:
- The brain-gut axis is a proven bidirectional communication link that contributes to the pathogenesis of dementia.
- Dementia is now known to be characterised by chronic gut inflammation and leaky gut, contributing to neurodegeneration and cognitive decline.
- The Dementia Support OTF was developed to combat the chronic oxidative stress that underlies dementia by boosting the nitric oxide synthetase pathway.
Methods
- Preclinical rats and murine ethics approval obtained in the Animal Laboratory of Ahmadu Bello University, Zaria, Nigeria, with the approval of the Ahmadu Bello University Committee on Animal Use and Care (ABUCAUC), with the Ethical Approval Number ABUCAUC/2025/0062.
- Scopalamine was used to induce both behavioural memory loss and hippocampus structural neurodegeneration changes in test models.
- Subacute Toxicity studies involving 28 days of dosing Dementia Support OTF and Probiotics in test Wistar rats with induced memory loss.
Subacute toxicity
- Maximum tolerable limit of OTF & Probiotics.
- Histologic and functional evaluation of vital organs via haematological and biochemical profiles post 21 to 28 days daily dosing of the agents, respectively.
Biomarkers screening
- In the Scopolamine-Induced memory loss murine model, the following factors are examined: BDNF, NGF, Beta-Amyloid 42, phospho-Tau 181 & 217, IL-1, Homocysteine, and Cholinesterase.
Behavioural tests
- Y Maze, Morris Water Maze, Elevated Plus Maze, Novel Object Recognition (NORT) Test, with a test for loss of memory and cognition.
Results
- Subacute Toxicity: The Maximum tolerable dose of OTF & Probiotics was >5000 mg/kg. Both products were non-toxic to the vital organs (structurally as well as functionally). All evaluated haematological and biochemical parameters were within normal limits. Histologically, no significant structural damage to any vital organ was evident.
- Biomarker Analysis (OTF): No effect on Beta-Amyloid-42 levels, Phospho-tau 181 levels reduced by 47 %, Doubled BDNF levels, Homocysteine levels reduced by 64 % and NGF levels increased by 11 %.
- Neurohistological Results (OTF): OTF at 5-100 mg/kg did not cause any visible histological alteration in the cortex. While PMCV 002 at other doses (250, 500, and 1000 mg/kg) showed only vacuolation of pyramidal cells.
- Behavioural Efficacy (OTF):
-
-
- Morris Water Maze: Pretreatment of rats with OTF (1000, 500, and 250 mg/kg) as well as Donepezil (5 mg/kg) significantly reduced the escape latency as compared to the scopolamine-alone group.
- Object Recognition Test: The exploration time significantly (p<0.05) increased in the standard group (Donepezil, 5 mg/kg) and all the tested doses of OTF. Significant (p<0.05) increase in discrimination with Donepezil (5 mg/kg) and OTF (250 mg/kg) as compared to scopolamine-alone.
- Preclinical Studies of Probiotics Development: Probiotics are superior to Donepezil in reducing key neurodegeneration biomarkers, including Beta-Amyloid-42, IL-1β, Phospho-Tau 181 & 217, Neurofilament light chain (NFL), and Cholinesterase levels, while increasing BDNF and NGF. In behavioural studies, probiotics proved to be superior in all four cognition and memory tests (Y Maze & Elevated Plus Maze, Escape Latency and NORT tests). Key markers of oxidative stress were also evaluated during the preclinical development of the probiotics, revealing a significant increase in glutathione, superoxide dismutase, and malondialdehyde, but had no marked effect on catalase levels.
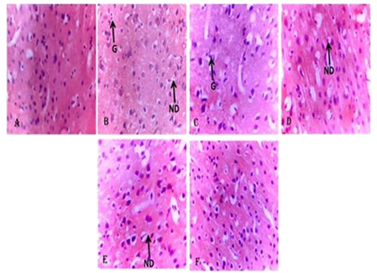
Fig. 1. Photomicrograph of the Cortical Sections of the Brain Showing the effect of PMCV002 after Scopolamine-induced Amnesia in Mice. (a). Distilled water group showing normal cortex (b). Scopolamine 1 mg/kg group showing severe gliosis and neuronal degeneration (c). Donepezil 5 mg/kg showing mild gliosis (G) (d). PMCV 002 at 1000 mg/kg showing mild neuronal degeneration (ND) (e). PMCV002 at 500 mg/kg showing mild neuronal degeneration (ND) (f). PMCV 002 at 250 mg/kg showing normal features. Image Credit: Image courtesy of Adedapo Adesokan et al., in partnership with ELRIG (UK) Ltd.

Fig. 2. The Dementia Support OTF package. Image Credit: Image courtesy of Adedapo Adesokan et al., in partnership with ELRIG (UK) Ltd.

Fig. 3. OTF on the tongue. Image Credit: Image courtesy of Adedapo Adesokan et al., in partnership with ELRIG (UK) Ltd.

Fig. 4. Demetia Support Probiotics. Image Credit: Image courtesy of Adedapo Adesokan et al., in partnership with ELRIG (UK) Ltd.
Conclusion
- This preclinical set of safety and efficacy studies demonstrated that probiotics proved superior to Donepezil in reducing Beta Amyloid-42, IL-1Beta, and Cholinesterase levels, and increasing BDNF. In contrast, OTF was superior to Donepezil in increasing BDNF and NGF levels, as well as decreasing Phospho-Tau 181 and 217 levels.
- These key subacute toxicity, relevant biomarkers, strategic cognition, and memory behavioural tests have shown that OTF and Probiotics are not only safer and non-toxic in the brains of memory loss modelled test animals compared to Donepezil, but also more efficacious.
- Based on the premise that dementia and cognitive impairment in the elderly are well-documented to carry a high morbidity burden, after successful human trials, OTF and Probiotics would offer viable adjunct dementia treatment options in the not-too-distant future.
- The next step would be a trial of both products in demented patients in the UK and the US.
About Precisemed Limited
Precisemed Limited specializes in the development of novel Over-the- counter (OTC) oral medicines, topical patches and gels.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free-of-charge to attend!
Our values
Our values are to always ensure the highest quality of content and that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world class conferences, networking events, webinars and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Dec 3, 2025