Human papillomavirus (HPV) infection is the major cause of cervical cancer. Hybrid capture based human papillomavirus detection is a test that can detect different types of human papillomavirus, which is used to detect high-grade cervical lesions.
Human Papillomavirus. Image Credit: Liya Graphics / Shutterstock
Human Papillomavirus and Cervical Cancer
Human papillomavirus (HPV) is a common cause of sexually transmitted disease which has no immediately noticeable symptoms in the early stages of infection, so the rate of incidence is higher than figures suggest. HPV is associated with many clinical conditions ranging from lesions to cancers. There are many different strains of HPV that can cause different diseases, but ~30 types are associated with the formation of cervical carcinomas and other closely linked diseases.
Infections with the HPV are oncogenic and lead to the development of cervical carcinomas and the cervical cancer precursor, cervical intraepithelial neoplasia (CIN). There are certain types of HPV that increase the risk of cervical cancer development, which are placed into the high-risk HPV category. Whereas, the HPV types that do not increase the risk of cervical carcinoma development are placed into the low-risk HPV category. The presence of high-risk HPV DNA is highest among sexually active teenagers.
Infections with multiple types of HPV can occur, which will increase the severity of cervical cancer. Most of the multiple infections contained two HPV types, but some contained three to five types of HPV.
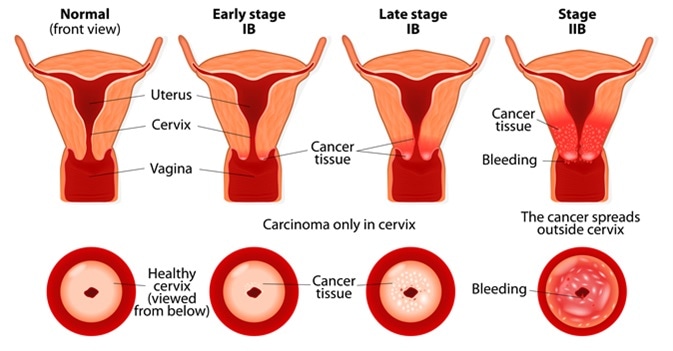
Cervical cancer stages. Image Credit: Designua / Shutterstock
Pathogenesis of Human Papillomavirus
HPV only infects basal cells of stratified squamous epithelium as other cell types are heavily resistant. The HPV replication cycle begins with the entry of the virus into the basal layer of epithelium cells. Once inside the cell, HPV DNA replicates as the basal cell differentiates and begins to move to the surface of the epithelium. The basal cell differentiates into a keratinocyte in the suprabasal level of the epithelium. Here the virus begins to replicate and amplify its DNA causing viral assembly. The HPV uses host machinery for gene transcription and replication.
Hybrid Capture-Based Human Papillomavirus Detection
HPV DNA detection can be used to screen for the presence of cervical carcinomas, especially following the treatment for CIN. It has been previously proved that individuals with no high-risk HPV DNA present in a cervical sample are very unlikely to develop cervical cancer. Secondary HPV testing can be used to determine if an individual with unclear or low-grade cytological abnormalities is likely to develop cervical cancer based on the HPV DNA expression they exhibit.
The hybrid capture II (HCII) test is a nucleic acid hybridization assay, in which specimens that contain target DNA will hybridize with a specific HPV RNA probe. The probe mixture contains probes for each different type of low-risk and high-risk HPV.
The DNA-RNA hybrids are captured on a microplate coated with antibodies which are specific for the DNA-RNA hybrids. The antibodies are conjugated with alkaline phosphatase and a substrate which allow for their detection. The detection of the signal emits a light which is measured in a luminometer as a unit of relative light units (RLU). The samples are classed as positive for HPV DNA if their RLU is over 1.0, which is equivalent to 1 pg of HPV DNA per ml.
In conclusion, the sexually transmitted human papillomavirus has a great link to the development of cervical carcinomas and its precursors. Testing for the presence of human papillomavirus provides a very accurate screening method for cervical cancer. With more research into the hybrid capture testing methods, more information will be able to be gained from them. Also, more research into human papillomavirus using hybrid capture techniques will provide more information regarding diagnostics and treatment.
Further Reading
Last Updated: Sep 3, 2018