This article and associated images are based on a poster originally authored by Delia Brustur, Jennifer Borthwick, Peng Chen, Carina Davies, Ping Huang, Jeff Kennedy, Christian Kuper, John Maclean, Daniel Moseley, Benjamin Rahemtulla, François Saint-Dizier, Owen Smith, Zixuan Tong, Marta Westwood, Han Zang, Qionglin Zhang, and Pengfei Zhang, and presented at ELRIG Drug Discovery 2025 in affiliation with Pharmaron UK Ltd and Pharmaron.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Approach
- A review of Pharmaron’s fragment library highlighted that improvements could be made regarding structural diversity and physicochemical coverage.
- The fragment library was updated to enhance coverage of novel functionalities currently employed in drug discovery.
- The performance of the new library and platform was evaluated in a case study against BRD4.
1. Fragment selection workflow
- Automated calculation of properties, metrics, and similarity score
- Tailored Spotfire dashboard enables idea prioritisation and decision-making
- Fragment sociability and synthetic trackability assessment ensure rapid access to follow-up
- Ideas refined to improve the quality of designs
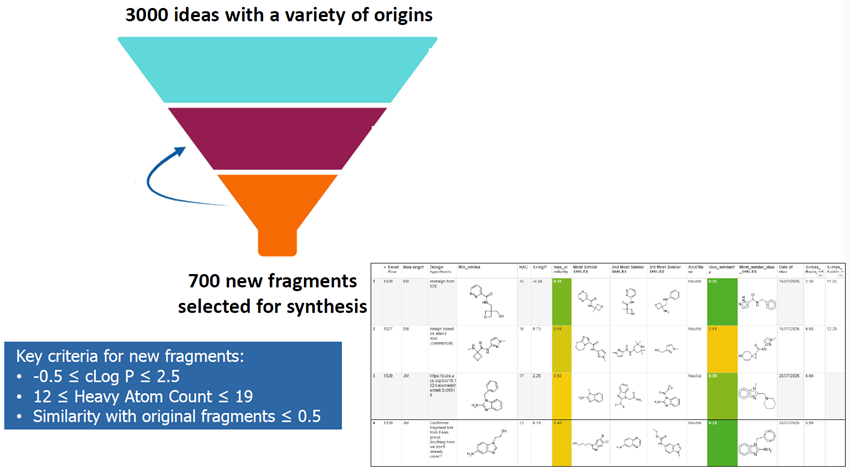
Image Credit: Delia Brustur et al., in partnership with ELRIG (UK) Ltd.
Key criteria for new fragments:
- -0.5 ≤ cLog P ≤ 2.5
- 12 ≤ Heavy Atom Count ≤ 19
- Similarity with original fragments ≤ 0.5
2. QC process
- A rigorous QC process was set up to ensure all added fragments were soluble and stable upon repeated freeze-thaw cycles in DMSO at -20 °C
- Criteria purity for new fragments to enter the QC process > 95 % by LCMS and NMR
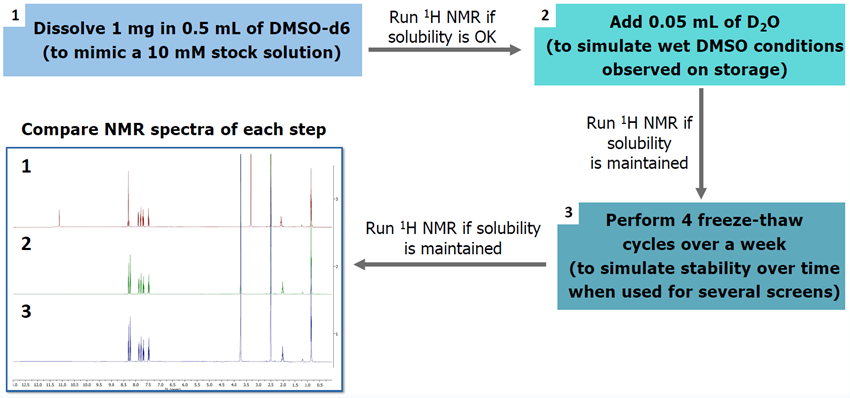
Image Credit: Delia Brustur et al., in partnership with ELRIG (UK) Ltd.
3. Visual results of library expansion
- Original library showed an opportunity to add fragments with low log P and higher heavy atom count (HAC) → “area to enrich”
- Area identified for high solubility fragments with a higher hit rate
- 550 new fragments have been added → ~30 % into the “area to enrich”
- Pharmaron library was enriched without increasing key properties beyond the desired limits
- The enhanced library is much more diverse – a few of the new fragments have close analogues in the original library
- The library was updated with historical and emerging chemical functionalities and biological targets, such as:
- Phosphine oxides, sulfoximines, oxamides, BCP, -CF2H / RNA binders, molecular glues …
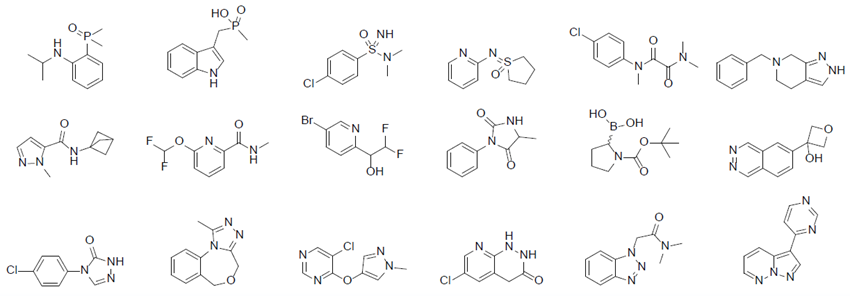
Image Credit: Delia Brustur et al., in partnership with ELRIG (UK) Ltd.
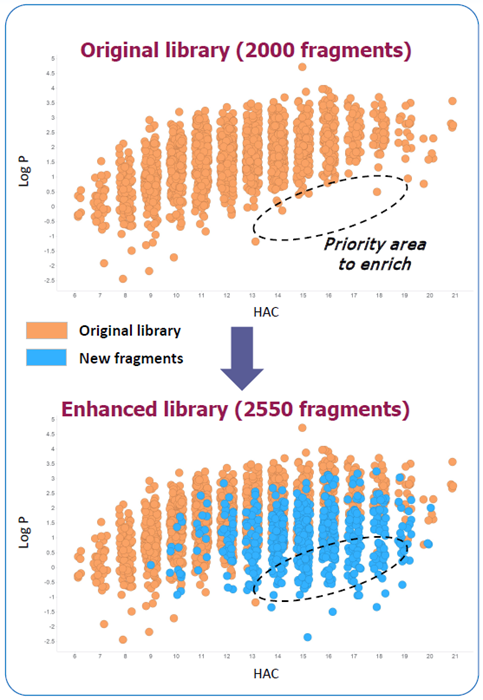
Image Credit: Delia Brustur et al., in partnership with ELRIG (UK) Ltd.
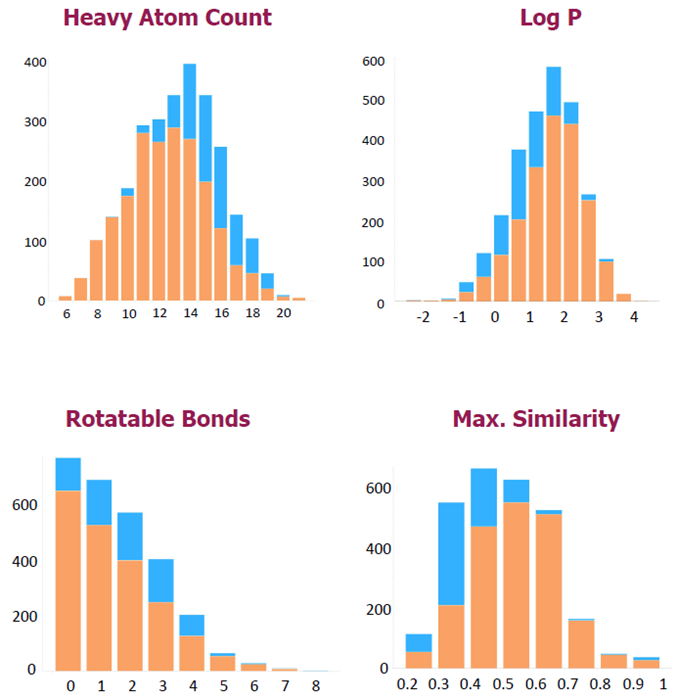
Image Credit: Delia Brustur et al., in partnership with ELRIG (UK) Ltd.
4. Case study: New library screened against BRD4 by SPR
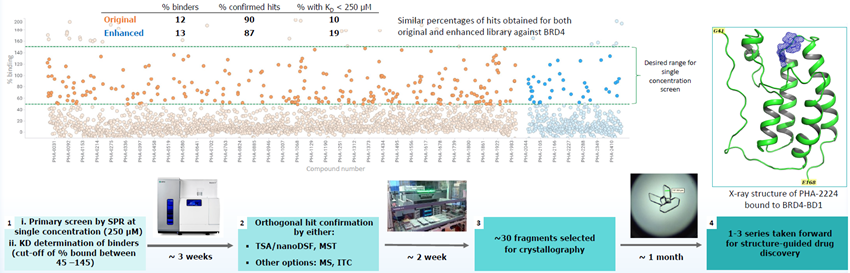
Image Credit: Delia Brustur et al., in partnership with ELRIG (UK) Ltd.
Conclusion
- The library was expanded by 25 %, with 550 new fragments added.
- A thorough selection workflow and QC process ensured new fragments possessed the desired diversity, novelty, and chemical stability.
- A comparison between new and original fragments was performed using SPR and found a similar hit rate, with higher-quality fragments.
About Pharmaron
Pharmaron (Stock Code: 300759.SZ/3759.HK) is a premier R&D service provider for the life sciences industry. Founded in 2004, Pharmaron has invested in its people and facilities, and established a broad spectrum of research, development and manufacturing service capabilities throughout the entire drug discovery, preclinical and clinical development process across multiple therapeutic modalities, including small molecules, biologics and CGT products
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free-of-charge to attend!
Our values
Our values are to always ensure the highest quality of content and that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world class conferences, networking events, webinars and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Nov 13, 2025