This article and associated images are based on a poster originally authored by Nermeen Ali, Heather Coleman, Azza Taher, Marwa Sarg and Noha Hilmy Elnagdi and presented at ELRIG Drug Discovery 2025 in affiliation with Ulster University, Cairo University, October 6 University, Al-Azhar University and Modern University for Technology and Information.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used to relieve pain and inflammation by inhibiting the cyclooxygenase (COX-1 and COX-2) enzymes involved in prostaglandin synthesis 1.
However, NSAIDs can have many adverse effects, including gastrointestinal bleeding 2. Some examples of pyrazole derivatives, such as Deracoxib, Ramifenazone, and Pyraclonil, have been reported as potent NSAIDs.3
Objective
To rationally design, synthesize, and biologically evaluate a new series of pyrazole-pyrazoline derivatives as structural analogues of Celecoxib, Indomethacin, and Floctafenine. The aim is to develop selective COX-2 inhibitors possessing enhanced antiinflammatory and analgesic efficacy and improved safety profiles.
Method
Novel pyrazole-pyrazoline derivatives were synthesized via the Vilsmeier–Haack reaction followed by condensation and cyclization steps to yield amide and ester analogues. Structures were confirmed using elemental analysis, IR, ¹H NMR, and MS spectroscopy.
Anti-inflammatory activity was assessed by the carrageenan-induced paw oedema test, while analgesic activity was evaluated with the acetic acid-induced writhing test in rats. Molecular docking was performed on the COX-2 enzyme (PDB ID: 4Z0L) to evaluate binding interactions and rationalize biological results.
Results
Most of the newly synthesized derivatives showed significant anti-inflammatory and analgesic activities compared with Indomethacin. Compounds 14b, 15b, and 22 produced the highest oedema inhibition (28.6-30.9 %) and analgesic effects (up to 84.5 % inhibition).
Docking studies revealed that compound 14b exhibited the strongest COX-2 binding affinity (-16.39 kcal/mol), forming key H-bonds with Arg120, Val349, and Ala527. The correlation between docking scores and in vivo activity supports their potential as selective COX-2 inhibitors
Graphs
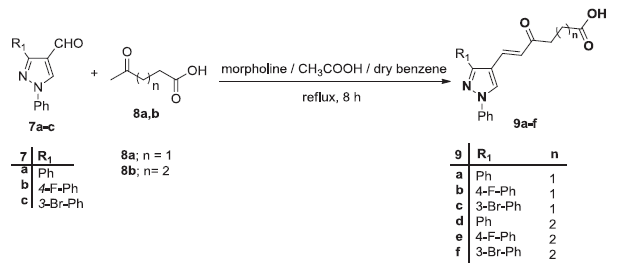
Preparation of the pyrazole carboxylic acids 9a-f. Image Credit: Image courtesy of Nermeen Ali et al., in partnership with ELRIG (UK) Ltd.
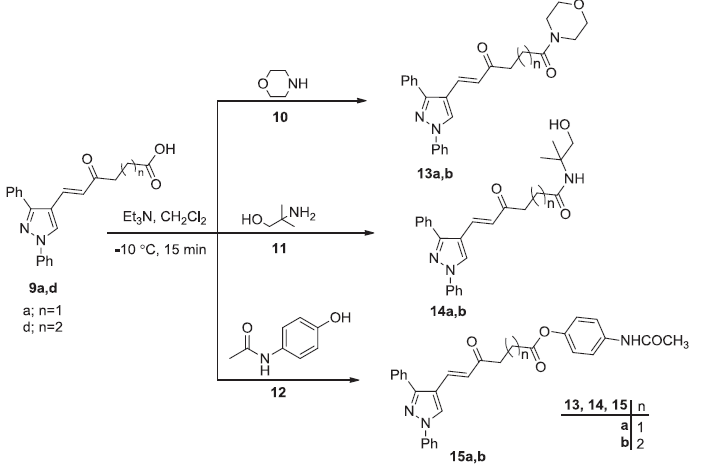
Preparation of pyrazole amides 13a,b, 14a,b and pyrazole esters 15a,b. Image Credit: Image courtesy of Nermeen Ali et al., in partnership with ELRIG (UK) Ltd.
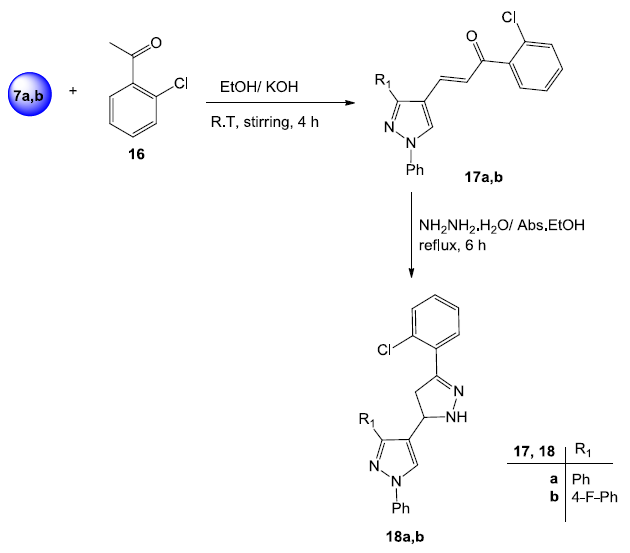
Preparation of the pyrazole chalcones 17a,b, and bipyrazoles 18a,b. Image Credit: Image courtesy of Nermeen Ali et al., in partnership with ELRIG (UK) Ltd.
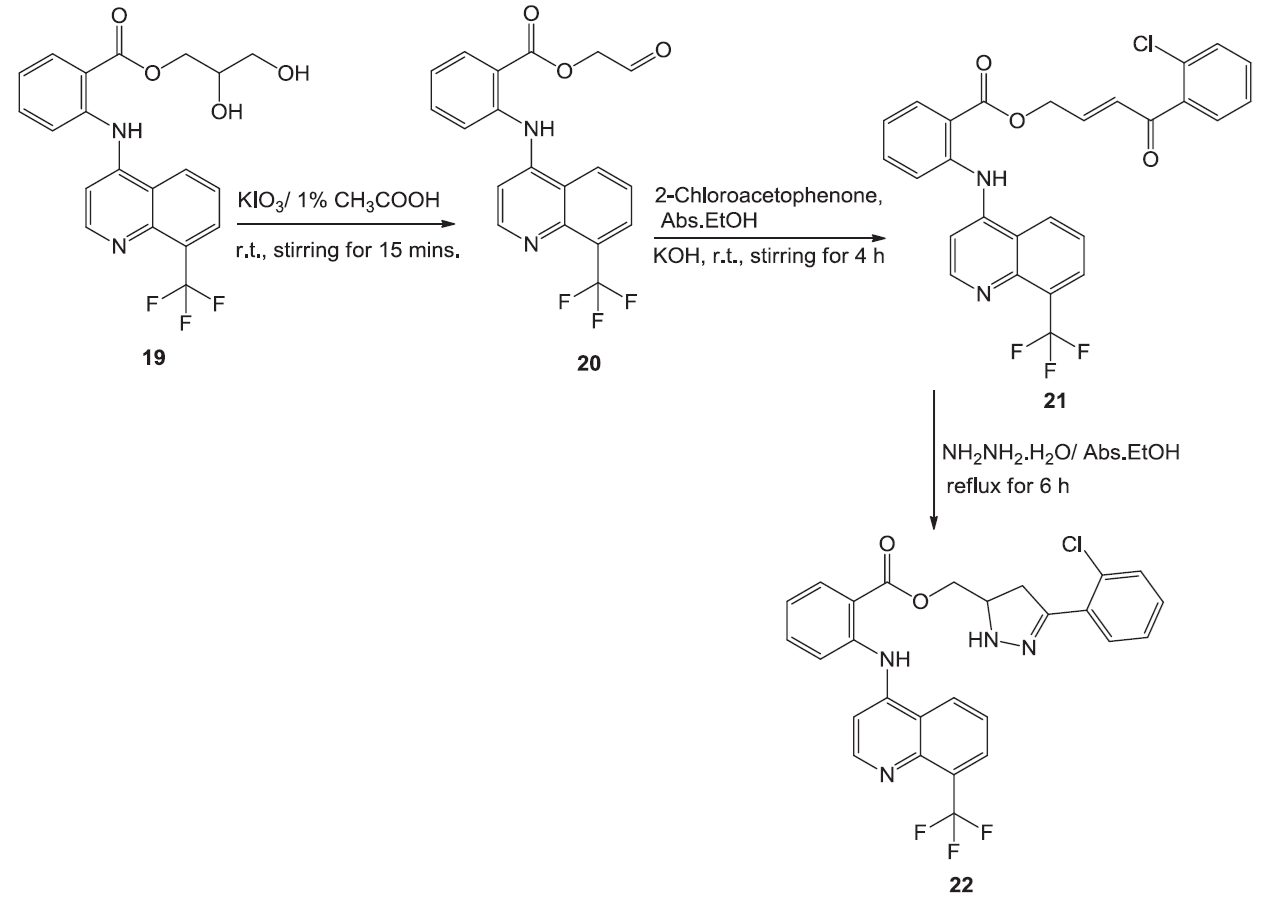
Preparation of Floctafenine chalcone 21 and pyrazoline 22. Image Credit: Image courtesy of Nermeen Ali et al., in partnership with ELRIG (UK) Ltd.
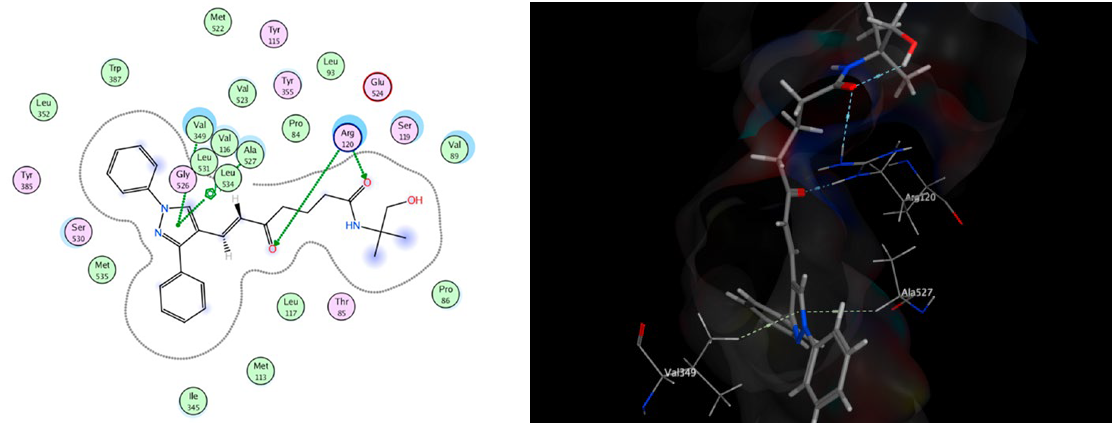
Docking of 14b into COX-2 (4Z0L). Image Credit: Image courtesy of Nermeen Ali et al., in partnership with ELRIG (UK) Ltd.
Conclusion
Structure-activity relationship (SAR) evaluation demonstrated that extending the alkyl chain and incorporating amide or ester functionalities significantly enhanced the anti-inflammatory and analgesic profiles.
Compounds 13b, 14b, and 15b with longer carbon chains exhibited superior anti-inflammatory activity (28.6-30.9 % inhibition), whereas pyrazoline analogues 18 and 22 showed the highest analgesic response (up to 84.5 % inhibition).
Cyclization to pyrazolines and fusion with a quinoline scaffold improved both potency and COX-2 selectivity. Molecular docking corroborated the experimental findings, identifying compound 14b as the most active through key interactions with Arg120, Arg513, Ser119, Val349, and Ala527 within the COX-2 active site.
References
- Agrawal, N. (2025). A Comprehensive Review on the Advancements of Dual COX‐2/5‐LOX Inhibitors as Anti‐Inflammatory Drugs. Chemical Biology & Drug Design, 105(5), pp.e70114–e70114. DOI: 10.1111/cbdd.70114. https://onlinelibrary.wiley.com/doi/10.1111/cbdd.70114
- Joshi, G.P., Henrik Kehlet and Lobo, D.N. (2024). Nonsteroidal anti-inflammatory drugs in the perioperative period: current controversies and concerns. British Journal of Anaesthesia. DOI: 10.1016/j.bja.2024.10.018. https://www.sciencedirect.com/science/article/abs/pii/S0007091224006524.
- Ghoneim, M.M., et al. (2024). Review of the recent advances of pyrazole derivatives as selective COX-2 inhibitors for treating inflammation. Molecular Diversity. DOI: 10.1007/s11030-024-10906-9. https://link.springer.com/article/10.1007/s11030-024-10906-9.
About Ulster University
Ulster University has a national and international reputation for excellence, innovation and regional engagement and continues to make a major contribution to the economic, social and cultural development of Northern Ireland.
Ulster has four campuses across the Northern Ireland - Belfast, Coleraine, Jordanstown and Magee. The university believes in making higher education accessible for all students.
Ulster’s core business activities are teaching and learning, widening access to education, research and innovation and technology and knowledge transfer.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics, and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free of charge to attend!
Our values
Our values are to always ensure the highest quality of content, that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer-led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate, and collaborate on an open-access basis. We achieve this through the provision of world-class conferences, networking events, webinars, and digital content.
Sponsored Content Policy: News-Medical.Net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.
Last Updated: Nov 26, 2025