From SelvitaReviewed by Olivia Frost
This article and associated images are based on a poster originally authored by Paulina Chorobik, Maciej B. Olszewski, John Vincent and Kirsty Winn and presented at ELRIG Drug Discovery 2025 in affiliation with Selvita.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Abstract
Target engagement is a key step in early-stage drug discovery, offering valuable insight into a compound’s efficacy and mechanism of action. This is typically assessed by measuring how well a drug binds to its intended therapeutic target. To make these findings more meaningful, it’s important to evaluate target engagement within a cellular and disease-relevant context - one that includes the appropriate cytoplasmic mediators and proteins. Adding this biological complexity helps ensure the results reflect real-world conditions.
Measuring compound engagement with a validated target early in the screening cascade provides a strategic advantage, as it can accelerate the identification and prioritization of promising drug candidates. Here, a proprietary Chemical Protein Stability Assay (CPSA) is described that directly measures drug–target interactions in cellular lysates. Comparable to other market alternatives, CPSA is designed to be simple, cost-effective, and scalable for broader application.
Introduction
CPSA is a distinctive technology licensed to Selvita from Medicines Discovery Catapult (MDC).
After exposing cells or lysates to compounds of interest, the protein target is treated with a chemical denaturing agent, and the proportion of the protein in its folded and denatured states are assessed. If the compound has bound to the target, the protein will become more stable with higher tolerability of a chemical denaturant. This leads to a shift in the denaturant concentration response curve compared to a suitable control and indicates that the protein and the compound are bound to one another.
Experimental workflow
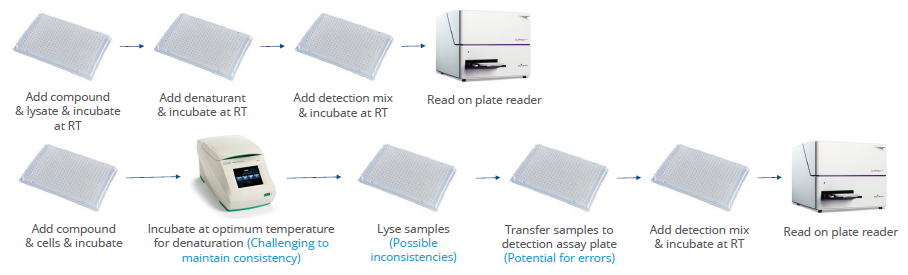
Figure 1. CPSA is a plate-based mix and read assay, compatible with automation and scalable to both 384 and 1536-well formats. Few steps make it simple to run, the use of lysates reduces variability and removes any need for protein generation, and there is no requirement for specialist equipment or high temperature incubations. It is also inexpensive to use, making it fully amenable to HTS. Image Credit: Image courtesy of Paulina Chorobik et al., in partnership with ELRIG (UK) Ltd.
(MTS data is not shown but is available here: https://md.catapult.org.uk/publications/mdc-chemical-protein-stability-assay-cpsa-a-novel-target-engagement-approach/)
Comparison to an alternative assay
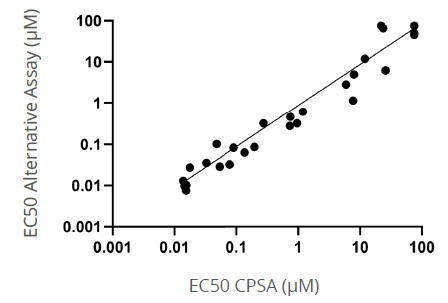
Figure 2. EC50 comparison between CPSA and an alternative thermal denaturation assay. Target engagement to p38 was assessed for the same compound set in both chemical (CPSA) and thermal (commercially available alternative) denaturation assay formats. Cellular lysates were screened in a 384-well format. There was significant correlation between the two technologies (r = 0.79 p=,0.0001). Data courtesy of Medicines Discovery Catapult. Image Credit: Image courtesy of Paulina Chorobik et al., in partnership with ELRIG (UK) Ltd.
Target engagement can be measured with different detection methods
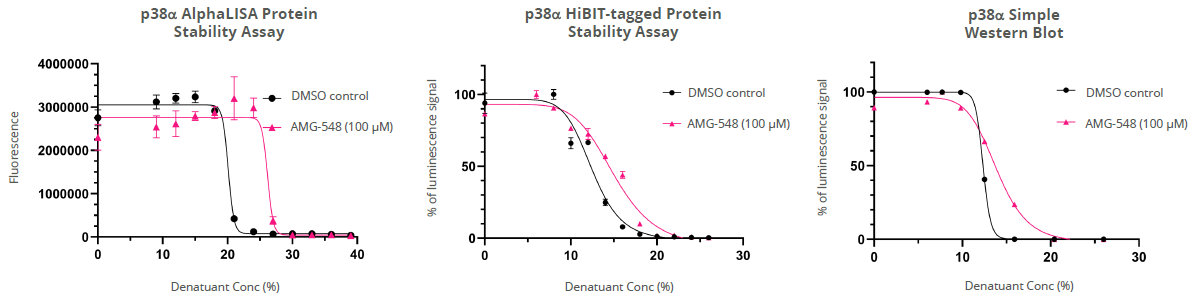
Figure 3. Measurement of protein stability in multiple detection technologies. Target engagement to p38α was assessed in cellular lysates in a multi-well format. Binding of AMG-548 to p38α shifted the proportion of the folded to denatured protein compared to the DMSO control. This potency shift was detected by AlphaLISA, HiBiT and Western blot technologies. Denatured proteins disrupt the mechanism of action of detection technologies, resulting in disruption to protein proximity measurements or antibody binding. Here a different chemical denaturant was used for each technology and a final denaturant concentration determined for subsequent experiments. Image Credit: Image courtesy of Paulina Chorobik et al., in partnership with ELRIG (UK) Ltd.
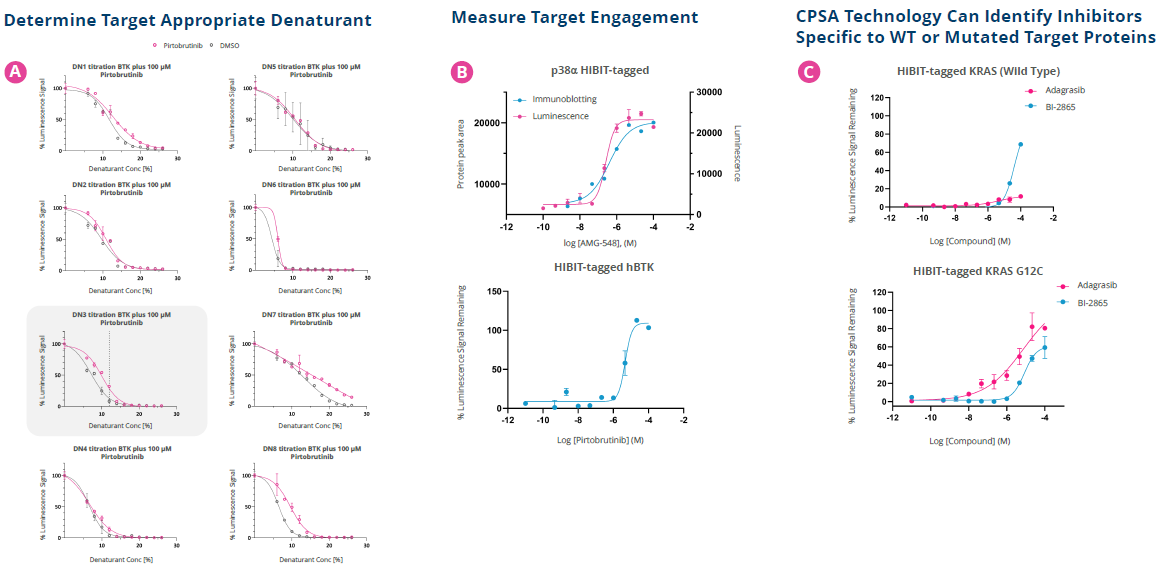
Figure 4. Application of CPSA to multiple cellular targets. Lysates were generated from HeLa (p38) or HEK cells (BTK & KRAS) overexpressing HiBiT-tagged target proteins. For each target, the optimal chemical denaturant type and concentration was determined. [A] shows an example of this for p38. Assays were run in 96-well (p38) or 384-well (BTK & KRAS) microtiter plates with the appropriate denaturant. Protein binding was measured via the Nano-Glo® HiBiT Lytic Detection System. [B] Target appropriate control compounds were screened in concentration response to determine pXC50s. There was parity between XC50s independent of the detection technology used. [C] Two inhibitors of KRAS were screened against HiBiT tagged WT protein or the G12C mutation. Adagrasib is specific for the KRAS G12C mutation and BI-2856 is a pan-RAS inhibitor. CPSA differentiated between the two, with target engagement absent for Adagrasib in the WT cell lysates. Image Credit: Image courtesy of Paulina Chorobik et al., in partnership with ELRIG (UK) Ltd.
Summary
CPSA is a target engagement technology that has demonstrated comparable pharmacology and data quality to commercial temperature-driven protein stability assays - for the targets tested. Its cost-effectiveness, streamlined HTS-compatible workflow, and scalability make CPSA a practical and efficient screening tool for assessing cellular target engagement in drug discovery programs.
Selvita has successfully applied CPSA across multiple protein targets and continues to collaborate with Medicines Discovery Catapult to broaden its applications. Together, this research aims to support researchers in making confident, data-driven decisions as they progress new therapeutic candidates.
About Selvita
Selvita is a leading European CRO supporting pharma and biotech companies with flexible, science-driven R&D services. We offer integrated or standalone solutions in drug discovery, preclinical studies, and GMP/GLP-compliant analytical development. With strong expertise across early to late development stages, we help accelerate new therapies by delivering high-quality data that enables smart decisions and faster time to market.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free-of-charge to attend!
Our values
Our values are to always ensure the highest quality of content and that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world class conferences, networking events, webinars and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Nov 13, 2025