This article is based on a poster originally authored by Adrian Pereira and Marie Croft.
Introduction
Accelerator mass spectrometry (AMS) is a highly sensitive analytical method for detecting 14C.
AMS was originally used for radiocarbon dating, but it is now commonly used to analyze clinical samples.
Early AMS investigations were primarily experimental, with a focus on microdosing (Phase 0), but today there is a validated analytical technique for providing Phase 1 clinical data.1,2
- Typical AMS-enabled clinical research involves administering a low 14C dosage (microtracer), usually 1 µCi of radiolabeled medication.
- In a human absorption, distribution, metabolism, and excretion (hADME) study, a therapeutic dose of the 14C-microtracer is given, and AMS is used to calculate mass balance recovery and provide metabolite concentration data.
- AMS can also be used to measure parent drug concentrations. In the intravenous pharmacokinetic (IVPK) study design, an IV 14C-microtracer is given alongside a therapeutic (usually oral) dose.
- AMS allows for determining absolute bioavailability (F) in a single cohort, as opposed to a standard two-cohort cross-over design.
The apparatus and technology for AMS have changed over time. AMS instruments have shrunk in size, become more user-friendly, and become a more widely available 14C-detection technology.
Evolution of AMS instruments

Image Credit: Pharmaron
Ionplus LEA AMS with key components
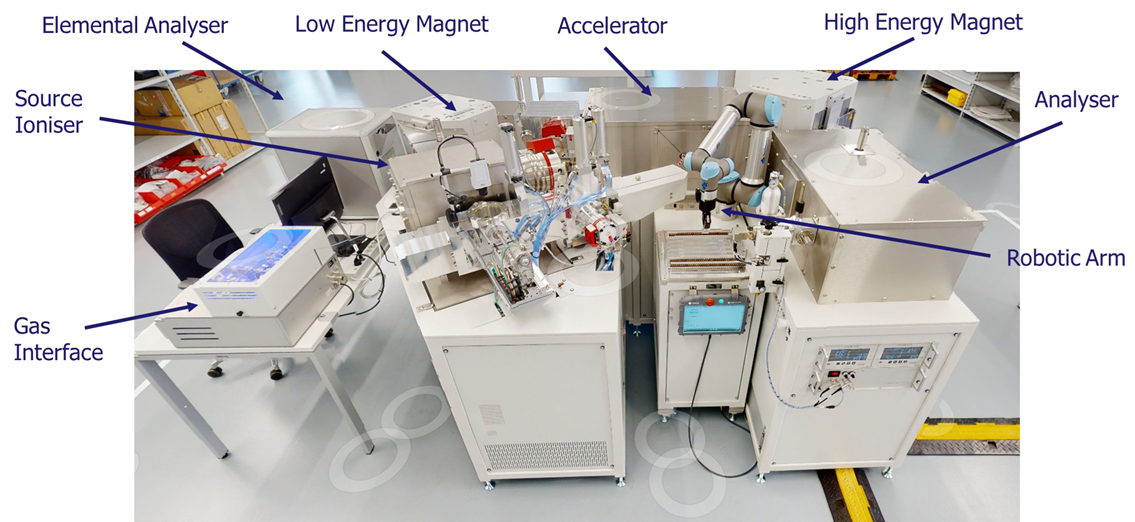
Image Credit: Pharmaron
Benefits of modern AMS technology
- Short sample processing times
- Interfaced analysis of samples
- Smaller footprint
- User-friendly, intuitive solutions
- Compliant with software regulations
Pharmaron AMS experience
- 350 compounds supported by AMS
- 70 FDA-approved medications
- Four systems in three laboratories worldwide
- 160 Phase 1 clinical investigations since 2018
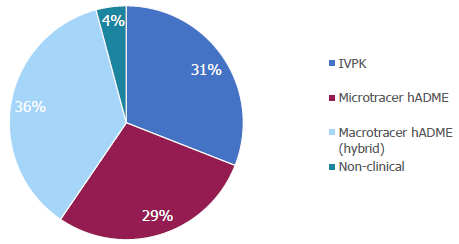
AMS studies by type (2018-2024). Image Credit: Pharmaron
AMS-enabled Phase 1 study designs
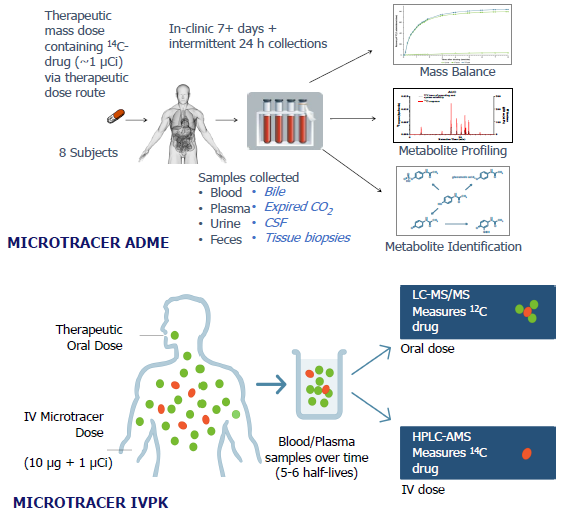
Image Credit: Pharmaron
Conclusions
- Modern AMS is a valuable technology for 14C-enabled clinical development.
- AMS instruments have become more user-friendly and accessible.
- LEA has the benefit of interfaced processing, allowing for faster sample analysis and data turnaround.
References
- Young, G., et al. (2022). Considerations for Human ADME Strategy and Design Paradigm Shift(s) – An Industry White Paper. Clinical Pharmacology & Therapeutics, 113(4), pp.775–781. DOI: 10.1002/cpt.2691. https://ascpt.onlinelibrary.wiley.com/doi/10.1002/cpt.2691
- Research, C. for D.E. and (2024). Clinical Pharmacology Considerations for Human Radiolabeled Mass Balance Studies. (online) U.S. Food and Drug Administration. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-pharmacology-considerations-human-radiolabeled-mass-balance-studies.
About Pharmaron
Pharmaron (Stock Code: 300759.SZ/3759.HK) is a premier R&D service provider for the life sciences industry. Founded in 2004, Pharmaron has invested in its people and facilities, and established a broad spectrum of research, development and manufacturing service capabilities throughout the entire drug discovery, preclinical and clinical development process across multiple therapeutic modalities, including small molecules, biologics and CGT products. With over 17,000 employees, and operations in China, the U.S., and the U.K., Pharmaron has an excellent track record in the delivery of R&D solutions to its partners in North America, Europe, Japan and China.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Jan 9, 2026