This article and associated images are based on a poster originally authored by William LaMarr, Dave Garby, Jeremiah Bowers, Lisa Boatner, Somayeh Talebzadeh, Aaron Wolfe, Sekar Ramachandran, and Peter Rye and presented at ELRIG Drug Discovery 2025 in affiliation with Momentum Biotechnologies and ICHOR Life Sciences.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Abstract
Chemoproteomic assays play a pivotal role in the discovery and development of small-molecule protein binders that act therapeutically through a covalent mechanism. In this work, the researchers presented the data for multiple complementary mass spectrometry-based assays, and the use of automation and multiplexing throughout, to investigate the covalent modification of KRAS G12C by inhibitor-14.
Collectively, the assays presented here address questions surrounding:
- If a protein of interest (POI) is covalently modified by compounds in electrophile libraries
- Which Cysteine sites on the POI are ligandable via covalent modification
- How much each reactive Cysteine site gets modified by a test compound
- How the potency of covalent binders can be rank-ordered using Kinact/Ki
- How compound binding to specific sites, for example, as part of SAR, can be quantified quickly using targeted acquisition methods
- The extent to which test compounds display on- and/or off-target binding in cells. Together, the portfolio of these streamlined workflows enabled the rapid identification and thorough assessment of covalent binding activity, including in vivo target engagement, for inhibitor-14 on KRAS G12C. Therefore, the culmination of data presented here exemplifies the promise of automation, multiplexing, and chemoproteomics in the contested pursuit of covalent ligand drugs.
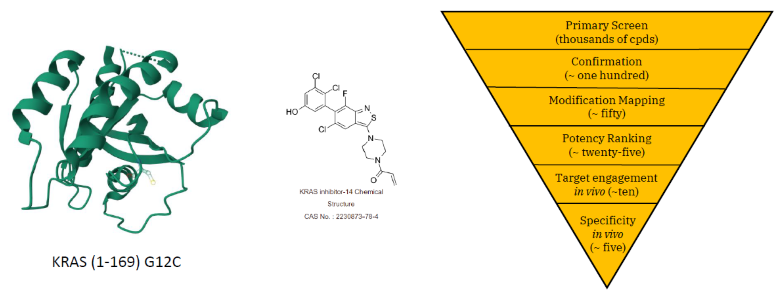
Image Credit: Image courtesy of William LaMarr et al., in partnership with ELRIG (UK) Ltd.
Assessing suitability of the target protein
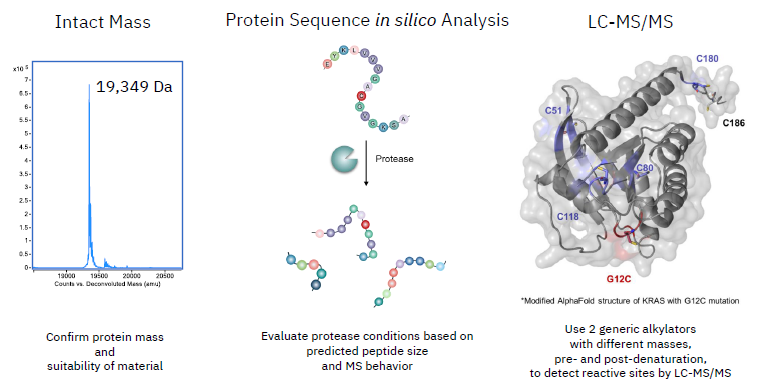
Image Credit: Image courtesy of William LaMarr et al., in partnership with ELRIG (UK) Ltd.
- KRAS G12C was of high quality and fit for ligandability studies by mass spectrometry techniques.
- Separate digestions by Trypsin, Chymotrypsin, and Elastase were found to cover all but one (Cys 186) possible sites.
- Protein found to have two reactive Cys sites, Cys 12 and 118.
- Trypsin digestion was suitable for measuring possible modification of both reactive sites.
Covalent binder ID by high-throughput library screening
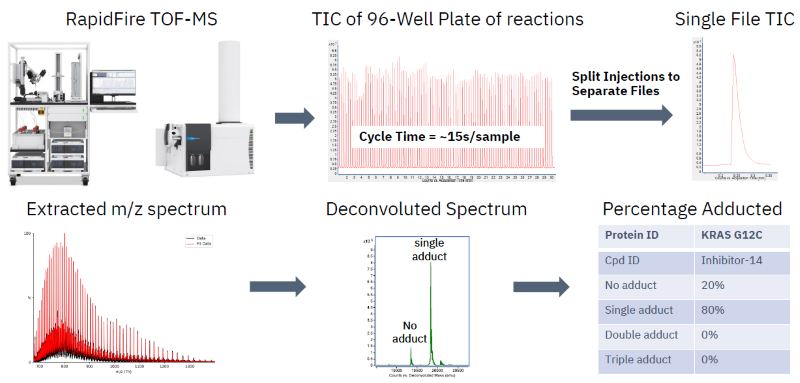
Image Credit: Image courtesy of William LaMarr et al., in partnership with ELRIG (UK) Ltd.
- RF-TOF MS workflow utilized intact mass analysis to find covalent binders to KRAS G12C and derive their respective adduct distributions.
- Inhibitor-14 was identified as the most potent binder for subsequent studies.
Identification of compound modification site and occupancy – Traditional method
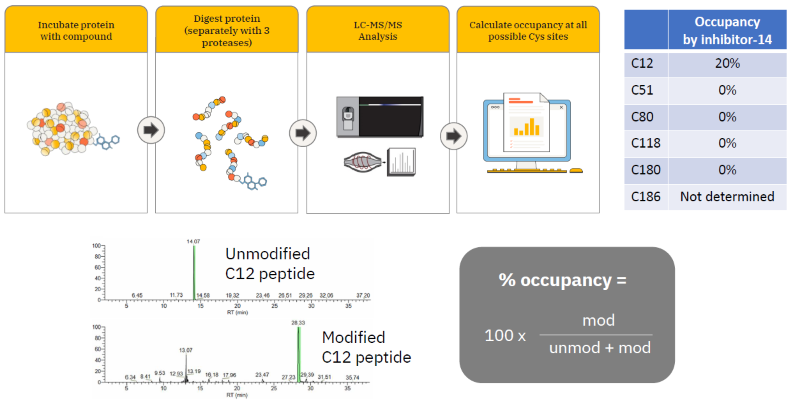
Image Credit: Image courtesy of William LaMarr et al., in partnership with ELRIG (UK) Ltd.
- KRAS C12 was the only reactive Cys site modified by inhibitor-14.
- 20 % occupancy was calculated, consistent with intact mass analysis data.
Identification of compound modification site and occupancy – 2-channel method
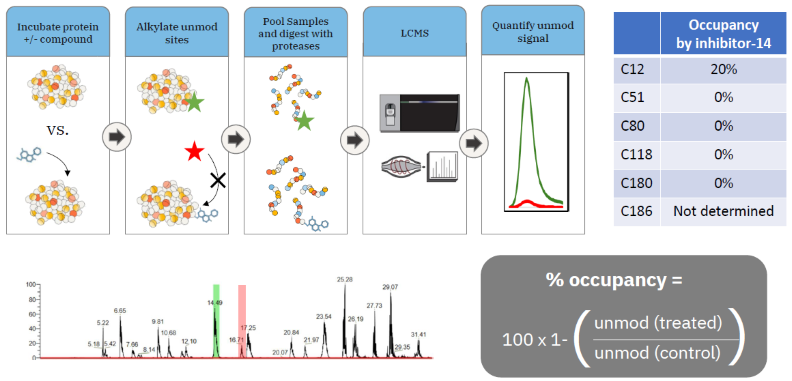
Image Credit: Image courtesy of William LaMarr et al., in partnership with ELRIG (UK) Ltd.
- The 2-channel method provided the same information as the traditional method, but with faster acquisition time and an easier analysis pipeline.
- More reliable than the traditional method for low and high occupancy modifiers.
Compound binding potency determination by Kinact/Ki
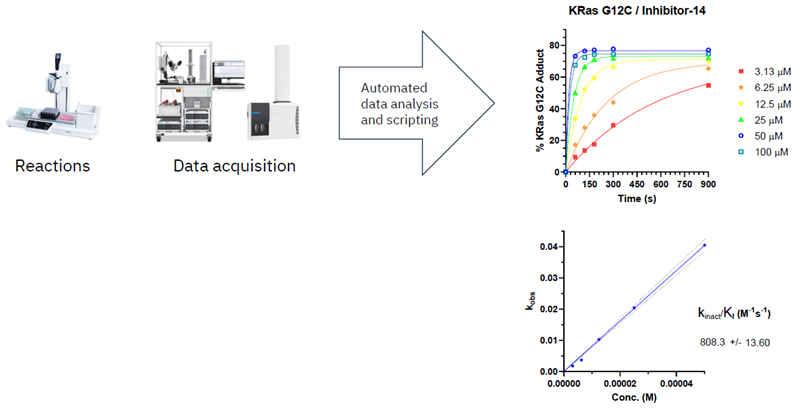
Image Credit: Image courtesy of William LaMarr et al., in partnership with ELRIG (UK) Ltd.
Kinact/Ki is a rate constant describing the efficiency of covalent bond formation.
Intact MS measured protein modification for multiple compound concentrations and time points.
Modification rates were plotted against the compound concentration
Apparent Kinact/Ki (M-1s-1) was interpolated from the slope
- Kinact/Ki values are independent of concentration and incubation time, making their determination suitable for interpreting SAR and translating compound activity from biochemical assays to the cell.
Studying on-target engagement in lysates/cells
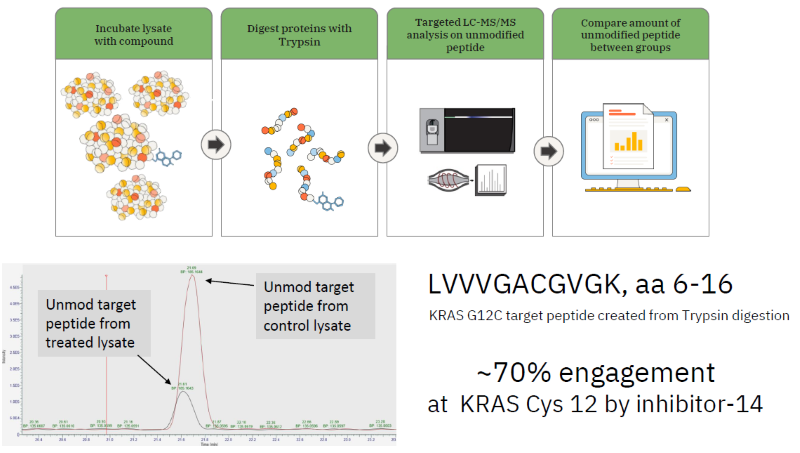
Image Credit: Image courtesy of William LaMarr et al., in partnership with ELRIG (UK) Ltd.
- Target engagement by covalent inhibitors can be inferred from the levels of unmodified target peptides before and after treatment.
- Sample preparation methods are automated on a KingFisher Flex System, enabling quick comparisons between multiple compounds and conditions.
Studying engagement specificity in cells
Proteins targeted by Inhibitor 14
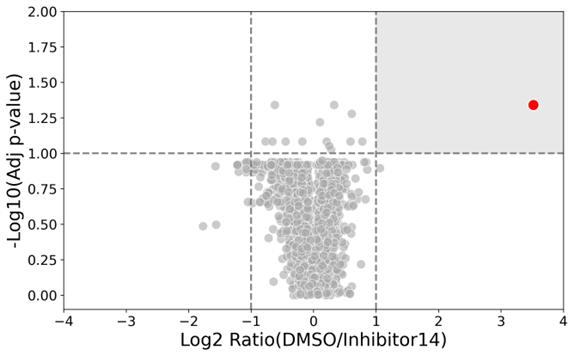
Image Credit: Image courtesy of William LaMarr et al., in partnership with ELRIG (UK) Ltd.
Proteins targeted by KB05
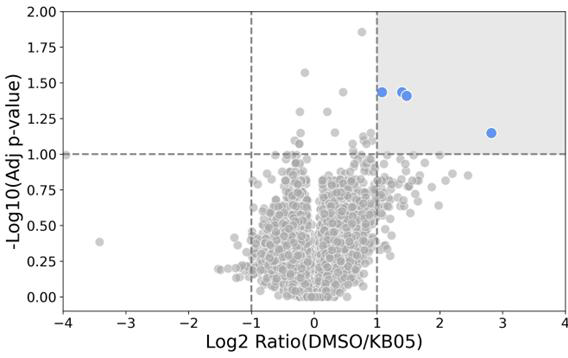
Image Credit: Image courtesy of William LaMarr et al., in partnership with ELRIG (UK) Ltd.
- Activity-based protein profiling was performed in cells with inhibitor-14 and KB05, a less specific covalent modifier.
- One significant modification event was observed for inhibitor-14 to KRAS C12 (left panel, red dot)
- Four significant modification events were observed for KB05 (right panel, blue dots).
Summary
- The irreversible binding of KRAS G12C by inhibitor-14 was used as a model system to highlight the power of mass spectrometry (MS) based assays for studying covalent inhibitors.
- Within this work, multiple assays are described that showcase how to find covalent hits from electrophile libraries, determine the hit binding site and occupancy, rank compound binding potency, measure target engagement in vivo, and interrogate binding specificity in complex mixtures.
- ≥ 5,000 compounds a day can be screened for protein-specific activity using a combination of automated sample preparation, the RapidFire high-throughput MS platform, and data analysis automation routines
- Target-specific projects can be taken from primary screening through to validation of selective target engagement in cells and tissues in ≤ 6 weeks.
About Momentum Biotechnologies
Momentum Biotechnologies (“Momentum”) is a specialized mass spectrometry services provider and discovery partner supporting clients in the pharmaceutical, biopharmaceutical, biotechnology, gene therapy, and toxicology sectors. The company’s core team of scientists and engineers has collaborated since 2000, when they developed the RapidFire technology (later acquired by Agilent in 2011), and has been serving clients using RapidFire-MS since 2004.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programs that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free-of-charge to attend!
Our values
Our values are to always ensure the highest quality of content and that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world class conferences, networking events, webinars and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Nov 26, 2025