Introduction
Mechanism of Action
Life Science Innovation
Therapeutic Frontiers
Challenges and Future Directions
Conclusions
References
Further Reading
Molecular glues represent a new generation of small molecules that harness the cell’s own degradation machinery to remove harmful or “undruggable” proteins. This article examines their structural basis, discovery methods, and emerging clinical promise in oncology and beyond.
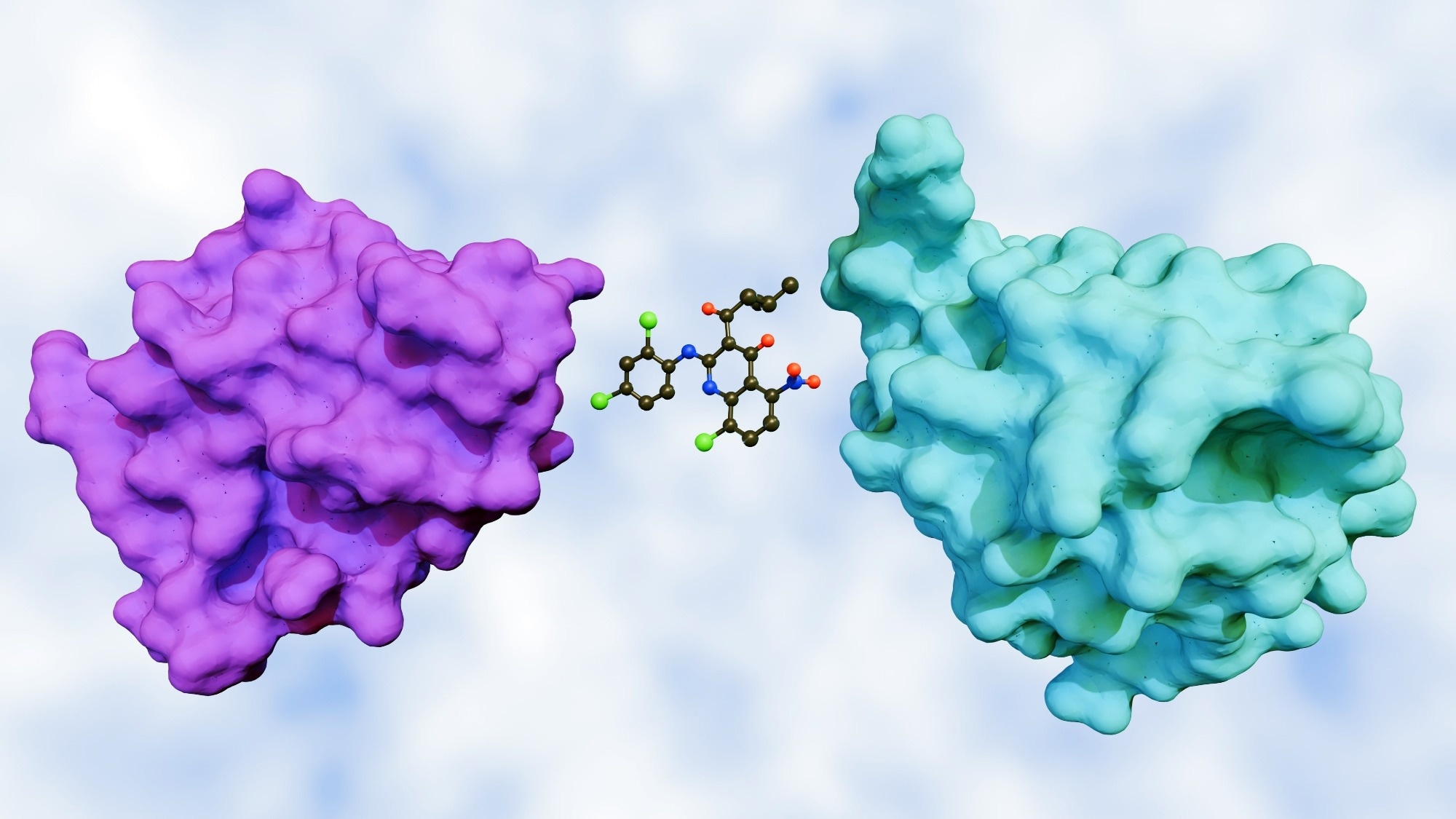
Image credit: Love Employee/Shutterstock.com
Introduction
Molecular glues are small, monovalent molecules that reshape an E3 ubiquitin ligase (E3) surface to promote a new protein-protein interaction with a target, triggering ubiquitination and removal by the ubiquitin-proteasome system (UPS). In contrast, proteolysis-targeting chimeras (PROTACs) are bifunctional molecules that bridge a ligand for an E3 to a ligand for the protein of interest via a linker to form a ternary complex.
Glues are typically smaller (often more drug-like) and can enhance proximity without a linker, whereas PROTACs offer modularity. Molecular glues act catalytically and can function even when the target lacks an active site or pocket, broadening the scope of “undruggable” proteins. Both strategies enable the catalytic knockdown of proteins, expanding drug discovery into the “undruggable” proteome, which lacks conventional binding pockets.1,5
This article demonstrates how molecular glues recruit E3 ligases to degrade undruggable proteins, contrasting them with PROTACs. It outlines the mechanisms, including Artificial Intelligence (AI)-driven design, clinical applications in cancer and other diseases, and challenges in selectivity, resistance, and safety.
Mechanism of Action
Molecular glues are small molecules that co-opt an E3 ubiquitin ligase to bring a target protein into a ternary complex, positioning lysines for ubiquitin transfer and proteasomal removal. Unlike bifunctional PROTACs, glues are monovalent binders that remodel an E3 surface to stabilize a new protein-protein interaction. In the cereblon (CRBN) system, thalidomide-like glutarimide scaffolds anchor in a hydrophobic, tri-tryptophan pocket of CRBN’s thalidomide-binding domain, with the glutarimide carbonyls and amide forming key hydrogen bonds. Substituents on the phthaloyl ring dictate which neosubstrates are recruited.
Lenalidomide exemplifies this selectivity: by binding to CRBN, it promotes the recruitment of Ikaros family zinc finger protein 1 (IKZF1)/ Ikaros family zinc finger protein 3 (IKZF3), as well as, in specific contexts, casein kinase 1α (CK1α). This ligand-dependent reprogramming defines CRBN as both a drug target and substrate receptor within the CRL4CRBN complex.1,2
CK1α recognition depends on a β-hairpin degron (residues 35–41) whose glycine 40 (G40) is critical for the CRBN-lenalidomide interface, explaining why closely related analogs differ in substrate profiles. Structural studies confirm that the β-hairpin forms a recurring “G-motif” degron that underlies CRBN's substrate selectivity across multiple glues. Celgene Compound-885 (CC-885) shows how adding an extended phthalimide-like moiety changes neosubstrate preference: the added extension protrudes from the CRBN pocket to contact a β-hairpin in G1-to-S-phase transition 1 (GSPT1), also known as eukaryotic release factor 3a (eRF3a), enabling its ubiquitination and degradation, an activity not seen with classic immunomodulatory drugs (IMiDs).
Overall, glutarimide-based CRBN glues catalytically trigger target knockdown by stabilizing a cooperative ternary complex with Cullin-RING ligase 4-cereblon (CRL4CRBN), resulting in the polyubiquitination and proteasomal clearance of disease-relevant proteins.1,2,3
Life Science Innovation
High-resolution structural and computational tools now map the ternary geometry of the E3 ubiquitin ligase, the molecular glue, and the target, exposing degron-centered hotspots that mediate selectivity. Cryo-EM, X-ray crystallography, and AlphaFold Multimer reveal how E3–target interfaces stabilize through short β-hairpin motifs, while machine-learning models predict new degron sequences and ligase–substrate compatibilities. AlphaFold Multimer predicts interfaces between E3 ligases and substrates, helping to nominate “sandwiched” interfacial pockets for stabilization.
Meanwhile, physics-based docking, molecular dynamics, and free-energy calculations refine poses and guide structure-activity relationships. In CRBN systems, structural analyses reveal a β-hairpin G-motif degron as a recurring signature that guides the exploitation of glues to reprogram substrate preference, clarifying how binding motifs drive ubiquitination. Together, these methods turn transient protein-protein interactions into designable sites for rational glue discovery.3,5
Fit-for-purpose assays and biophysics now de-risk hits and quantify cooperativity. Nanoluciferase and NanoLuc Binary Technology (NanoBiT) abundance assays enable high-throughput screening, with awareness of High-BiT (HiBiT) lysine-tag artifacts that can confound readouts. Nuclear magnetic resonance (NMR) spectroscopy identifies cryptic pockets and reports on dynamics, while isothermal titration calorimetry (ITC) and kinetics (association/dissociation) provide insight into efficient ternary assembly.
Fragment-based and structure-based design expand glue-like chemotypes beyond classical scaffolds and seed-focused libraries for further optimization. Cooperativity (α > 1) has emerged as a quantitative marker of effective ternary stabilization, strongly correlating with degradation potency in both CRBN and VHL systems. These assay and readout innovations, layered onto predictive models, provide a closed loop from virtual triage to experimental validation, accelerating the development of selective, drug-like molecular glues.3,5
Therapeutic Frontiers
Clinically validated molecular glues built on CRBN redirect the CRL4 to degrade the transcription factors Ikaros (IKZF1) and Aiolos (IKZF3), producing durable responses in multiple myeloma. Next-generation cereblon E3-ligase modulators (CELMoDs) deepen and accelerate IKZF1/3 loss while amplifying anti-tumor immunity. However, patient-derived mutations in CRBN and IKZF1/3 that disrupt lenalidomide binding can drive resistance, emphasizing the need for ligand analogs that preserve neosubstrate engagement despite CRBN variation.4
Programs are pushing into historically “undruggable” targets: Signal Transducer and Activator of Transcription 3 (STAT3) degraders show that proximity-induced removal can silence a central oncogenic node across solid and hematologic cancers, and discovery efforts are probing glue-like chemotypes for selective STAT3 engagement. Parallel work aims to disable the MYC proto-oncogene, basic helix-loop-helix leucine zipper (MYC) transcription factor, with early reports describing compounds that collapse MYC-dependent transcriptional programs and promote degradation in MYC-driven tumors.1,4,5
Targeted protein degradation (TPD) with glue scaffolds is being explored in neurodegeneration to clear intrinsically disordered, aggregation-prone proteins such as tau and alpha-synuclein, with emphasis on central nervous system (CNS) penetration and brain-relevant E3 ligases. In immunology, CRBN-directed glues reprogram lymphocyte transcription and cytokine networks, suggesting precision tuning of autoimmunity and inflammation without broad immunosuppression.
Emerging glues are also being developed for kinases (e.g., NEK7) and RNA-binding proteins (e.g., RBM39, RBM23), expanding degradation beyond transcription factors. Together, these advances position molecular glues as compact, drug-like agents for degrading transcription factors in oncology (IKZF1/3 today; STAT3 and MYC emerging) and as versatile tools for disease modification in neurodegeneration and immune modulation.1,3,4,5
Challenges and Future Directions
Off-target degradation and narrow selectivity remain central risks because non-selective target ligands can recruit multiple homologs. Two engineering levers help:
- E3 choice to create ligase-driven selectivity (for example, ring finger protein 114 (RNF114) versus CRBN or von Hippel-Lindau (VHL) can bias degradation of the breakpoint cluster region-Abelson fusion (BCR-ABL) versus cellular Abelson tyrosine kinase (c-ABL) when using the same kinase recruiter.
- Ternary-complex design to harness cooperative geometry so that only the intended paralog forms a productive complex. Selective degraders have been built from relatively broad-spectrum ligands for fibroblast growth factor receptor 1/2 (FGFR1/2) and STAT3/5 by exploiting these principles.
Cooperative ternary assembly and E3 expression bias in specific tissues are now viewed as key selectivity determinants.1,5
Resistance can emerge through target re-expression, E3 loss or down-regulation, and mutations. Degraders may retain activity against inhibitor-resistant mutants because event-driven degradation tolerates affinity loss better than occupancy-based inhibitors. Still, clinical bioavailability and CNS penetration, especially for large bifunctionals, remain hurdles. Molecular glues, with their smaller size and oral bioavailability, partially overcome these pharmacokinetic challenges but require careful profiling to avoid covalent or off-target E3 engagement.1,4,5
Discovery pipelines are shifting toward rational and computational workflows. AI, structure-based design, crystallography, in-silico docking, and phenotypic and biochemical screens are being integrated to mine degron motifs, model glue-stabilized interfaces, and virtually triage ligase–target pairs, reducing off-target risk and improving cooperativity. The integration of deep learning for degron prediction and virtual screening of E3-ligase diversity (CRBN, DCAF15, RNF114, VHL, KEAP1) marks the next phase of molecular glue design.
Future priorities include expanding the E3 toolkit, selecting tissue-biased ligases, and improving oral and brain-penetrant pharmacokinetics. Industry platforms from Monte Rosa and C4 Therapeutics demonstrate this computation-first trajectory toward the clinic.1,3,5
Download your PDF copy now!
Conclusions
Molecular glues emerge as next-generation small molecules that redirect endogenous quality control pathways for precision therapy. By remodeling an E3 interface to recruit disease drivers, they catalyze ubiquitination and clearance through the UPS, achieving catalytic knockdown beyond occupancy limits. Compared with PROTACs, glues are compact, orally bioavailable, and capable of binding to proteins lacking deep pockets.
Advances in structural biology, cryo-EM, and AI-driven ligand design are rapidly expanding their therapeutic range and improving selectivity. Progress in structural biology and AI is enhancing selectivity and reducing off-target risks. The field now shifts its focus to biased ligases, brain penetration, and resistance management to deliver durable degradation across cancer, neurology, and immunology.
References
- Sasso, J. M., Tenchov, R., Wang, D., Johnson, L. S., Wang, X., & Zhou, Q. A. (2022). Molecular glues: the adhesive connecting targeted protein degradation to the clinic. Biochemistry. 62(3). 601-623. DOI:10.1021/acs.biochem.2c00245, https://pubs.acs.org/doi/10.1021/acs.biochem.2c00245
- Ito, T., Yamaguchi, Y., & Handa, H. (2021). Exploiting ubiquitin ligase cereblon as a target for small-molecule compounds in medicine and chemical biology. Cell Chemical Biology. 28(7). 987-999. DOI:10.1016/j.chembiol.2021.04.012, https://www.sciencedirect.com/science/article/pii/S2451945621002051
- Kang, C., & Xu, W. (2025). Leveraging structural and computational biology for molecular glue discovery. Journal of Medicinal Chemistry. 68(3). 2048-2051. DOI:10.1021/acs.jmedchem.5c00076, https://pubs.acs.org/doi/10.1021/acs.jmedchem.5c00076
- Barrio, S., Munawar, U., Zhu, Y. X., Giesen, N., Shi, C.-X., Da Viá, M., Sanchez, R., Bruins, L., Demler, T., Müller, N., Haertle, L., Garitano, A., Steinbrunn, T., Danhof, S., Cuenca, I., Barrio-Garcia, C., Braggio, E., Rosenwald, A., Martinez-Lopez, J., Rasche, L., Raab, M. S., Stewart, A. K., Einsele, H., Stühmer, T., & Kortüm, K. M. (2020). IKZF1/3 and CRL4CRBN E3 ubiquitin ligase mutations and resistance to immunomodulatory drugs in multiple myeloma. Haematologica. 105(5). DOI:10.3324/haematol.2019.217943, https://haematologica.org/article/view/9410
- Bouvier, C., Lawrence, R., Cavallo, F., Xolalpa, W., Jordan, A., Hjerpe, R., & Rodriguez, M. S. (2024). Breaking Bad Proteins - Discovery Approaches and the Road to Clinic for Degraders. Cells. 13(7). DOI:10.3390/cells13070578, https://www.mdpi.com/2073-4409/13/7/578
Further Reading
Last Updated: Nov 28, 2025