Sponsored Content by AbselionReviewed by Olivia FrostNov 27 2025
In this interview, News Medical-Life Sciences speaks with Dr. Ruizhi Wang, Founder and CEO of Abselion, about simplifying the quantification of His-tagged proteins using the Amperia™ platform. Dr. Wang, discusses how electrochemical sensing, validated reagents, and user-focused workflows are helping researchers move from complex protocols to consistent, scalable measurement.
To begin, could you briefly introduce Abselion and your role in the development and application of the Amperia™ platform?
I’m the Founder and CEO of Abselion, a Cambridge-based life sciences technology company focused on simplifying biomolecule quantification. We develop practical analytical tools that help scientists generate reliable data without the complexity of traditional systems.
Amperia™ is a compact benchtop platform that uses electrochemical sensing to quantify proteins and viral vectors quickly and reproducibly. It combines tested chemistry, engineered sensors, and intuitive software into a single, integrated workflow. Our goal is to turn complex analytical tasks into straightforward measurements that can be run directly from crude or purified samples.
In my role, I lead Abselion’s overall strategy and product development, working closely with our R&D, production, and commercial teams to turn new ideas into dependable products. My background in physics and biosensors has shaped our approach to innovation. We apply engineering precision to biological systems to create tools that are robust, scalable, and genuinely useful for everyday research and development.
His-tagged proteins are widely used across various expression systems, yet quantification remains a challenge. What specific gaps or inefficiencies did Abselion aim to address with the Tagged Protein Quantification Kit?
Quantifying His-tagged proteins is a routine part of recombinant protein workflows, but obtaining reliable, comparable results across constructs and experiments can still be difficult.
Techniques such as gels or colorimetric assays are useful for quick checks, but they only offer relative insight and can vary with sample preparation or expression background. More quantitative approaches, while effective, often rely on purification or multi-step protocols that are difficult to apply in routine research.
The His-tag Protein Quantification Kit was developed to make this step more consistent and accessible. It provides a ready-to-use assay for quantitative analysis directly from crude or clarified lysates. The workflow is straightforward and reduces both handling steps and variability between users.
By removing the practical barriers to reliable His-tag measurement, the kit helps researchers focus on data interpretation rather than setup. It enables more confident comparisons across constructs, conditions, and stages of development, and supports a more data-driven approach to protein research.

Image Credit: Abselion
Can you explain how the Amperia™ platform's redox electrochemical detection method differs from traditional quantification techniques like ELISA or spectrophotometry, especially in terms of performance in crude samples?
Traditional techniques, such as ELISA or spectrophotometry, rely on optical measurements, which can be affected by turbidity, color, or background absorbance. These are common issues when working with crude lysates.
Amperia™ uses a redox-based electrochemical detection method. Instead of relying on light, the platform measures an electrical signal generated by a defined redox reaction on the sensor surface, which directly reflects the amount of target bound.
Because Amperia™ does not rely on optics or fluidics, it is less sensitive to sample interference and offers faster, more consistent measurements. Sensor handling and readout are automated within the instrument, which improves reproducibility and reduces manual steps that often introduce variability in conventional assays.
The new assay operates using a premix competition format. Could you elaborate on how this design simplifies workflows and enhances reproducibility for end users?
The assay uses a premix competition format, where the sample containing His-tagged proteins is mixed with a labeled detection reagent prior to measurement. Both components compete for a fixed number of anti-His binding sites on the sensor surface.
When more target protein is present, it occupies more of the sites, leaving fewer available for the labeled reagent. This results in a lower signal that is inversely proportional to the target concentration.
This format eliminates the need for separate binding and washing steps, which simplifies the workflow and reduces variability. Because the target and detection reagent interact under the same conditions, the assay remains robust even with complex samples, such as crude lysates. It delivers consistent assay behavior across users, instruments, and expression systems.
The kit incorporates anti-His antibodies supplied by GenScript. How does their integration onto the sensor strips improve assay consistency and reduce manual intervention?
The His-tag Protein Quantification Kit was developed in collaboration with GenScript, whose THE™ His Tag Monoclonal Antibody technology is known for its high affinity and reliable recognition of His-tags across constructs and systems. By integrating this antibody directly onto Amperia’s sensor strips, we ensure consistent capture performance while maintaining broad compatibility with common tag configurations.
Each strip is pre-coated under controlled manufacturing conditions. This removes the need for users to handle or optimize reagents, significantly reducing assay-to-assay variability and improving reproducibility between users and sites.
How does the Amperia™ platform support scalability from early expression screening through to downstream development, and what benefits does this bring to bioprocessing teams?
Amperia™ brings a consistent approach to biomolecule quantification across the development pipeline, from early expression screening to purification and process optimization. While individual assays may vary in design, they follow the same operating principle and guided workflow. This makes it easy to generate comparable, high-quality data across multiple applications, including His-tag proteins, antibodies, and AAVs.
Scalability in this context is more about flexibility than throughput. Each automated run is quick and self-contained. Users can run a few samples or dozens, depending on project needs. This makes Amperia™ well-suited for early screening as well as in-depth process studies, without requiring new methods or retraining.
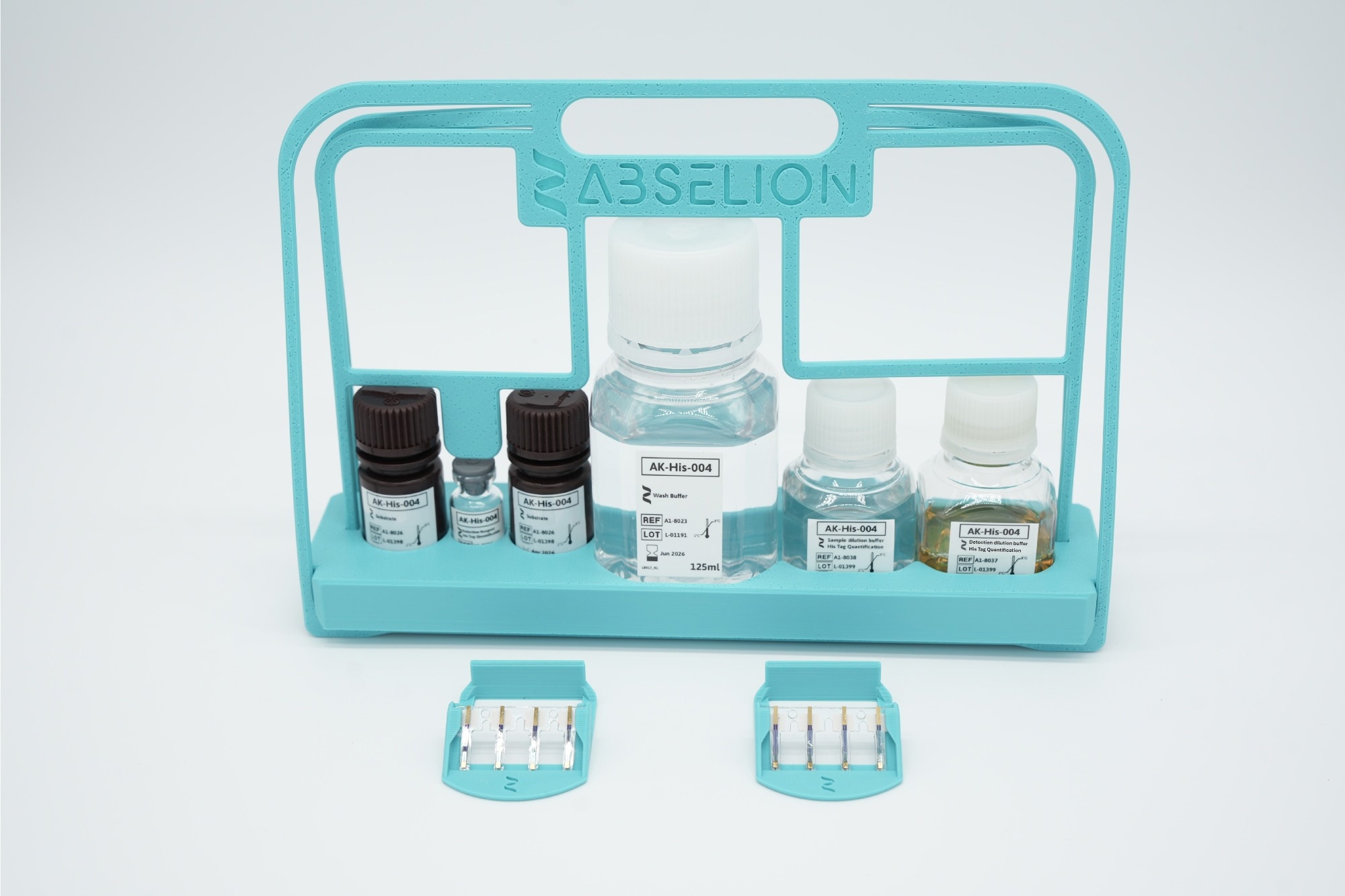
Image Credit: Abselion
In addition to the full quantification kit, Abselion has introduced a standalone sensor strip pack. What needs or user feedback led to this modular offering, and how do you see it being used in diverse lab settings?
The idea came directly from user feedback. Many researchers wanted more flexibility, either to extend existing assays or to explore custom applications using their own reagents. By offering standalone sensor strip packs, we enable users to select the configuration that best fits their workflow.
Different sensor types are available, depending on the application, including pre-coated antibody strips for quantification kits and functionalized surfaces, like streptavidin, for custom assay development. All sensor strips are manufactured and quality-controlled to the same standard as our kits.
Finally, how do you see this approach to His-tagged protein quantification shaping future trends in protein analytics and bioassay development?
We are seeing growing demand for tools that simplify protein analytics without compromising on data quality. Platforms like Amperia™ support that shift by reducing complexity, increasing reproducibility, and enabling faster decisions based on robust data.
As the field evolves, I expect more integration of validated reagents, automation, and thoughtful engineering. The future of protein quantification is not just about improving sensitivity or specificity. It is about making high-quality measurement accessible, efficient, and scalable across the entire R&D pipeline.
About Dr. Ruizhi Wang
Dr. Ruizhi Wang is the founder and Chief Executive Officer of Abselion, a Cambridge (UK)-based company developing practical analytical solutions for biologics and cell and gene therapy. He leads the company’s overall strategy, planning, and operations, with a focus on making advanced biomolecule analytics more accessible to researchers and industry users.
Ruizhi is an award-winning scientist and entrepreneur, recognised by the Royal Society of Chemistry, Merck Group, and Cambridge University Entrepreneurs. He is also a Royal Academy of Engineering Enterprise Fellow and a former scholar of the German Academic Merit Foundation.
He holds a PhD in Physics from the University of Cambridge and MSc/BSc degrees in interdisciplinary sciences from ETH Zurich. He has co-authored more than 20 scientific publications with over 700 citations.
Education & Qualifications: PhD, University of Cambridge; MSc/BSc, ETH Zurich.
Awards & Fellowships: Royal Academy of Engineering Enterprise Fellow; Royal Society of Chemistry Emerging Technologies Award; Merck Group Innovation Award; Cambridge University Entrepreneurs Award; German Academic Merit Foundation Scholarship.
Professional Memberships: Royal Academy of Engineering Enterprise Hub; Royal Society of Chemistry.
Areas of Expertise: Biosensors, electrochemistry, biomolecule analytics, process development, biologics and gene therapy workflows, technology translation, and entrepreneurship
About Abselion
Abselion was founded in 2018, at that time under the name HexagonFab, in a small corner of a laboratory at the University of Cambridge.
We set out with the goal to make protein research simpler. Scientists should be able to pursue their passion for discovery and innovation, rather than spend their valuable time on tedious, manual tasks. With RED, we had access to the ideal technology to create this product.
A product that is so compact that it could fit on every bench, and so affordable that it is accessible to everyone. Over the years, we have designed, built, and tested our first product, Amperia™, and we’re proud to introduce it to the world.