Matteo, what is a polymorph?
Polymorphism is the ability of a solid material to exist in more than one crystal structure. Solids tend to be ordered and different spatial three-dimensional arrangements of the same molecules can give us different crystal structures, so different polymorphs. Polymorphism is a tendency of polymers, minerals, metals, organic compounds and even pure elements (although, in this case we talk about allotropy rather than polymorphism).
Image Credit: SenSeHi / Shutterstock.com
In pseudopolymorphism, the different crystal types are the result of hydration or solvation. Therefore, in the crystal structure we don't just have our new chemical entity or new active pharmaceutical ingredient, but also solvents or water which take part of the order of the material meaning solvates and hydrates can exist in different crystal structures.
Recently, the pharmaceutical industry has paid a considerable amount of attention to non-ordered materials. At the solid-state, we can sometimes find and try to stabilize amorphous material. Similar to polymorphs, we find that amorphous materials can take on several modifications; this is called polyamorphism.
Why is the solid form of a drug so important to the pharmaceutical industry?
The Ritonavir story is good example of why the form has become so important in this industry. Ritonavir was a blockbuster medicine against HIV marketed in 1994 in a capsule formulation. Later, a solid to solid transition between Form I (as it was called at the time) and the not fully characterized Form II was observed.
Form II, a polymorph, had dramatically less solubility than Form I. This meant that patients who were supposed to take a capsule that would be effective against HIV, found that the medicine was no longer as effective. Ritonavir had to be temporarily recalled with an estimated loss of millions during the market absence of this drug product.
What is the Ritonavir case tell us?
The Ritonavir case is another example illustrating that different polymorphs can have different properties, such as thermodynamic stability and solubility, with a direct impact on the drug product performance in terms of bioavailability, exposure, solubility and stability. Therefore, the selection of the API form for drug product development is more than critical because the API form itself has a great impact on the properties of the final drug product: the form selection problem has ethic, therapeutics, commercial and economic implications.
What does the solid form have to do with patent expiration and line extension?
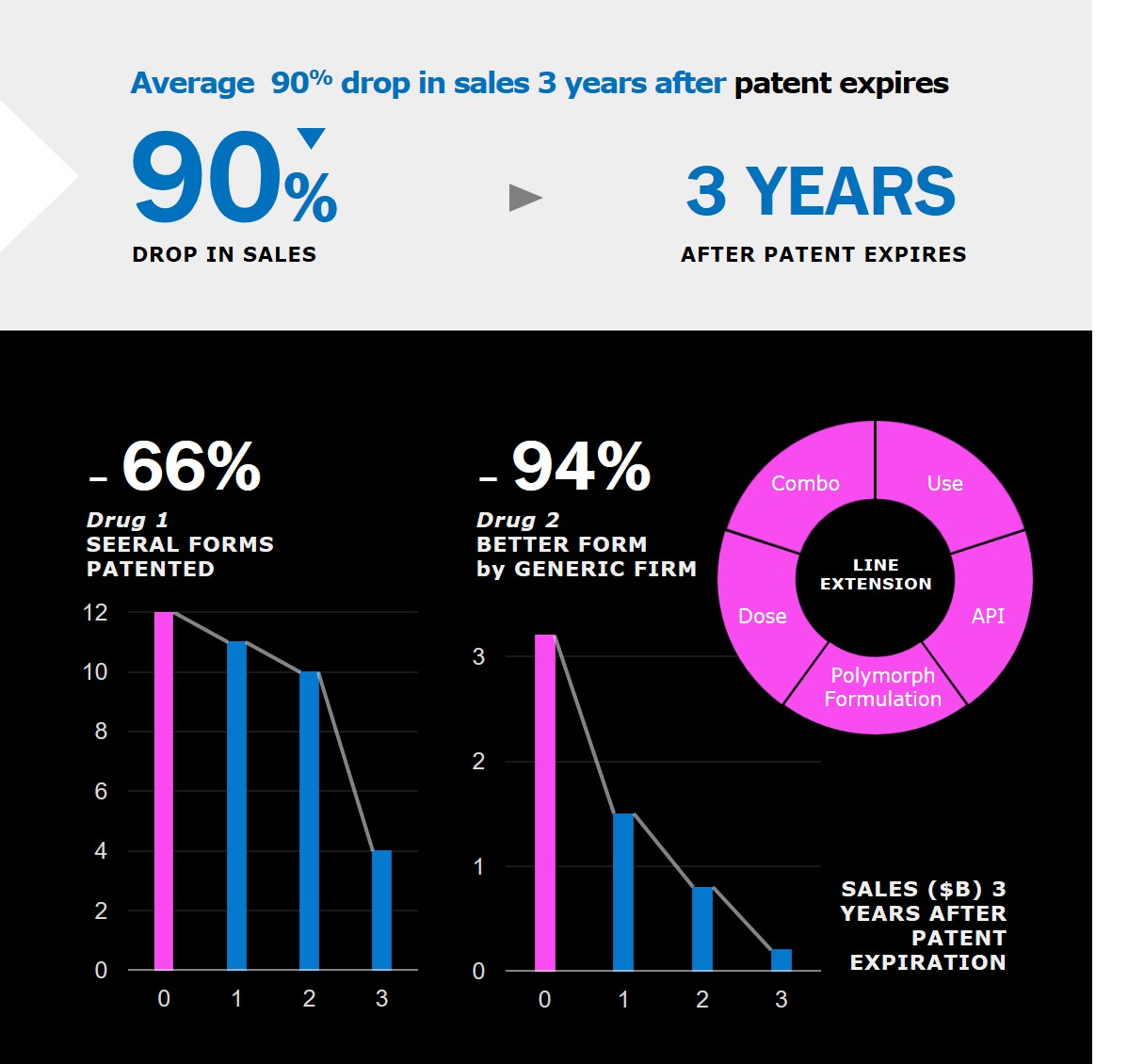
Patent expiration is a threat for pharmaceutical industry and an opportunity for generic companies. In average, there is a 90% decrease of revenue after the patent expiration of a drug. There are different strategies to line extension such as combination therapies, new medical applications, a new isomer, different dosing and new solid forms (crystalline or amorphous). Millions are spent by drug originators and generics firms on intellectual property lawsuits dealing with solid form. When this happens, you want the best data in your hands!
What techniques are used for solid form characterization?
There is a portfolio of analytical techniques to characterize and quantify solid-state aspects in terms of structural chemistry for our API. There are classical spectroscopy techniques like Raman and infrared, thermal analysis like differential scanning calorimetry (DSC), and more sophisticated techniques like neutron diffraction and electron diffraction and magnetic resonance techniques such as solid state NMR. Typically, we use more than one technique.
Why has powder X-Ray diffraction been considered the 'gold-standard' technique so far?
X-rays have a wavelength of the same order as the atomic spacing of atoms in a solid, meaning they interact with the electron density of the atoms in the coherent diffraction domain in the solid giving us information about the structure of the solid. You can determine the position of atoms, molecule position in what we call unit cell and the spatial group as depicted in the International Table of Crystallography.
X-ray powder diffraction is really our routine analysis or what we call the gold standard. It's easy to use in the lab (we have an in-house machine) and the most recent diffractometer has outstanding resolution. Also, due to some special optics, there is a reasonable acquisition time to have good data quality from both qualitative and quantitative method development.
We obtain a fingerprint that we qualitatively compare with a reference pattern. We can also develop quantitative methods because there is a linear relationship between the quantities of two polymorphs. We can reach a limit of detection of 1% or 2% with our in-house instrumentation. There are a large number of optics and detectors available on the market: in my view, the best consideration for pharma compounds is transmission geometry with a focusing X-ray mirror and a position sensitive detector.
How do you study amorphous materials using X-ray diffraction?
We study the pair distribution function (PDF); the function that describes the probability of finding two atoms separated by a certain distance in the material. This analysis gives us structural information from disordered materials.
The data set is more or less the same as an X-ray powder diffraction pattern, but we also take into account diffuse scattering. Therefore, we talk about the total scattering analysis in order to study the local atomic structure. The different amorphous phases can have different short-range order or arrangement. This type of analysis can be done in-house but we normally use a synchrotron radiation facilities to obtain data set for PDF analysis.
What do you do if you need high-resolution data?
If we need more resolution and data for specific applications, we can access synchrotron facilities to perform X-ray powder diffraction. This is where we perform X-ray diffraction using X-rays generated by a synchrotron. This radiation have very high brilliance and tunable energy that result in very high-resolution data.
 Image Credit: iviewfinder / Shutterstock.com
Image Credit: iviewfinder / Shutterstock.com
Sebastian, what is the role of magnetic resonance in the characterization and quantification of polymorphs?
Although Magnetic resonance is considered a well-established powerful technique to study polymorphs and amorphous forms, it has resided, so far, in the hands of few specialists. Recent technologic advances have enabled the deployment of the technique to a much wider scientific audience. We have overcome the price and complexity barriers, with the introduction of a benchtop spectrometer and greatly increase automation and sensitivity in our high-resolution floor standing instruments.
Can you tell us more about the new benchtop?
Yes, sure. A couple of years ago we introduced the minispec Form Check, a benchtop spectrometer for the quantification of components in solids mixtures such as a polymorph in the presence of another; amorphous form in the presence of polymorphs or polymorph/amorphous in the presence of excipients. The minispec Form Check approaches the quantification of solid forms from a completely different angle to that taken by other techniques (PXRD, Raman, IR). It is a time-domain (TD) instrument and therefore there is no fourier transform done, no frequency domain. The minispec Form Check pulls components apart based on their relaxation properties, T1 relaxation in this case. The T1 relaxation time of a drug's crystalline and amorphous form is generally very different, making this technique ideal, especially for the quantification of low level of amorphous (LOQ < 1%), which is challenging by PXRD and has a significant quantification error associated.
Being a TD-NMR technique, the minispec Form Check has also other pretty unique advantages such as being non-destructive and non-invasive: samples remain intact after measurement and they can be measured in their own container, in vials for example. It is a push-button solution, easy to use and a bench-top system with a low footprint, low price and low cost of ownership (no cryogens needed).
The minispec Form Check does what it says in the tin: quantification of one component in the presence of another, which can be a mixture. The instrument is calibrated with these two components before measuring the blends. Therefore, we are talking about quantifying components of well-defined systems. When we are not yet at the ‘control’ stage and the scientist is seeking to understand the problem, which is not yet well defined, then more sophisticated techniques, fourier transformed based, that tell us the full story, are required. Here is where solid-state NMR plays a crucial role, identifying structural changes and characterizing the different components in the drug mixture, a formulated drug, for example.
What are the improvements that have made open-access solid-state NMR a reality?
We recently introduced our new probe (detector) platform called the iProbe CPMAS. The iProbe platform, that also includes probes for liquid state and high resolution magic angle spinning (HRMAS) applications, provides full automation capabilities so we can automate the tuning and matching steps, needed when changing from one sample to another. On top of that, since this is solid-state NMR, precise adjustment of the magic angle spinning position is also needed. The iProbe CPMAS has a motor which accurately and precisely chooses the correct orientation, using KBr as a reference compound.
The probe is currently offered at 400, 500 or 600 megahertz (MHz) base frequency for standard bore systems. It comes in a double channel set-up, meaning we have a proton channel which is also tunable to fluorine, and we have a broadband channel which goes from phosphorus down to nitrogen. Spinning speed, for four-millimeter samples, is up to 15-kHz magic-angle spinning.
To work in a walk-up environment, we also need automation when changing samples. For this, we have a robot system that provides you up to 48 storage positions for four-millimeter rotors and a flexible tubing of up to ten meters connecting the storage to our NMR probe in the NMR system.
The robot does not need to be close to the magnet. One can have it in the preparation laboratory which could be separated from the NMR lab by a door, for example, and the flexible tubing (up to 10 meter) can be connected to the NMR system through a wall or through the ceiling.
To put the icing on the cake, the robot is now driven by our well-established IconNMR automation software, enabling the same workflow used in walk-up liquid state NMR laboratories for many years and eliminating any potential learning curve.
What about the new CryoProbe for solids?
We are also very proud to have introduced the first ever cryogenically cooled probe for solid-state NMR (CPMAS CryoProbe) which is especially useful for the analysis of very low amount of compounds, as it is the case of polymorphs/amorphous in formulated material (drug product). Cooled electronics and a cooled probe set-up, while keeping the sample itself at the desired experimental temperature, are responsible of a sensitivity increase of a factor of three to four, which translates in at least 1 order of magnitude productivity increase.
The probe is currently available in an HCN (proton, carbon, nitrogen) configuration for 600 MHz magnets and provides magic angle speed of up to 20-kilohertz with 3.2 mm rotors. It also provides high power decoupling and cross-polarization (CP) set-up, so one can use double CP or traditional CP where there's no limitation compared to room temperature probes. On top of that, the probe includes automatic tuning and matching as well as an automated set-up for the adjustment of the magic angle.
Altogether this package is enabling many experiments and applications which were not accessible before.
 Image Credit: Roger Costa Morera / Shutterstock.com
Image Credit: Roger Costa Morera / Shutterstock.com
Is the 600 MHz with a CryoProbe your most sensitive instrument?
No… You may, for example, need to analyze surface modifications or identify how a linker is bound to the API; the API is bound to an organic framework or API excipient interactions. For this, we can offer solid-state Dynamic Nuclear Polarization (DNP) which boosts the sensitivity even further. In optimum conditions one can achieve enhancement factors of up to 400, corresponding to a productivity gain factor of about 160,000.
Now, having heard this high number, please keep in mind that DNP, in general, is relying on an electron source and radicals added to the sample, indicating already that to use this technique one need to specially prepare the samples. Nevertheless, if one needs such sensitivity gains, this could well be the approach to get the answer, which might otherwise be impossible to reach.
The probes we are offering for DNP come in a double channel and triple channel set-up, HXY or HX probes, 1.3, 1.9 and 3.2 mm rotors with different spinning speeds, and we are offering the whole system at 400, 600 and 800 MHz magnetic field strength.
The probes provide cold inject and eject capability, meaning that we do not need to warm the probes to change samples or completely and manually remove the probe from the magnet. We can leave everything connected and just change the samples using a docking port.
Where can readers find more information?
About Dr. Matteo Daldosso
Matteo has a strong background in the solid-state characterization of pharmaceutical and inorganic materials. His major responsibilities focus on crystalline structuralization, API form, version selection, X-ray diffraction and scattering, thermal analysis, spectroscopy, regulatory compliance, and data integrity. Matteo has worked at Aptuit since 2010 and previously worked at GSK for four years, and before that at the ESRF, the European Radiation Synchrotron Facility, in Grenoble.
|
.jpg) |
About Dr. Sebastian Wegner
Sebastian studied physics at the University of Munster and conducted his Ph.D. in Physical Chemistry using solid-state NMR to characterize amorphous glass systems in melt and at room temperature. Sebastian joined Bruker in 2008 and he's now the Product Manager for the solid-state NMR.
|
.jpg) |
About Bruker BioSpin Group
The Bruker BioSpin Group designs, manufactures, and distributes advanced scientific instruments based on magnetic resonance and preclinical imaging technologies. These include our industry-leading NMR and EPR spectrometers, as well as imaging systems utilizing MRI, PET, SPECT, CT, Optical and MPI modalities. The Group also offers integrated software solutions and automation tools to support digital transformation across research and quality control environments.
Bruker BioSpin’s customers in academic, government, industrial, and pharmaceutical sectors rely on these technologies to gain detailed insights into molecular structure, dynamics, and interactions. Our solutions play a key role in structural biology, drug discovery, disease research, metabolomics, and advanced materials analysis. Recent investments in lab automation, optical imaging, and contract research services further strengthen our ability to support evolving customer needs and enable scientific innovation.