In this interview, industry experts Mathieu Bennet and Dustin Whitson explore how automation and single-cell technologies are transforming high-throughput B-cell cloning for antibody discovery.
Can you please introduce yourselves and your roles at Cellenion and Biosero?
Mathieu Bennet: I am the Chief Technology Officer at Cellenion. Our company was founded in 2016 as a spin-off from Scienion, a global leader in precision liquid dispensing. Our focus is on single-cell isolation and dispensing technologies. Since launching our first instrument in 2017, we have over 200 cellenONE systems installed globally, distributed through Scienion and partners. Since 2020, we have been part of the BICO group, allowing us to collaborate closely with sister companies like Biosero.
Dustin Whitson: I am an Application Consulting Scientist at Biosero. We develop science-centric software and automation solutions that support research labs worldwide. We have over 100 employees and 2,500 system installations across over 750 customers globally, including 15 top 20 pharma companies. Since 2021, we have also been part of the BICO group, which makes collaborations like this one with Cellenion possible.
Could you give us an overview of how monoclonal antibodies are developed and where B-cell cloning fits into this process?
Mathieu Bennet: The development of monoclonal antibodies began in 1975 with the hybridoma technique by Köhler and Milstein, a groundbreaking innovation that earned them the Nobel Prize. A few years later, in 1986, the FDA approved the first monoclonal antibody-based drug. These drugs treat cancer, autoimmune disorders, metabolic conditions, and infectious diseases today.
Several techniques allow monoclonal antibody development, the most common being hybridoma, phage display, and B-cell cloning. B-cell cloning is especially suited for therapeutics due to its speed and the diversity of antibodies it can generate. The downside is its complexity and cost. It demands skilled operators and often suffers from limited sample availability. We aim to simplify and scale this through automation.
Why is single-cell isolation important in B-cell cloning?
Mathieu Bennet: In B-cell cloning, single-cell isolation occurs at two crucial stages. First, during the initial screening phase, high-throughput isolation is required to analyze a broad antibody repertoire. Second, during the monoclonal selection stage, the best candidates for production are chosen.
Traditionally, fluorescence-activated cell sorting (FACS) is used. However, we have heard consistent feedback from users about limitations, particularly in cost, viability, and throughput. FACS requires skilled operators and costly consumables, and often compromises cell viability due to shear stress. Our cellenONE system was built to address all of these limitations.
Can you explain how the cellenONE technology works?
Mathieu Bennet: cellenONE is an image-based single-cell sorting and dispensing platform. It uses a high-resolution microscope and a glass capillary nozzle to gently isolate and dispense single cells. The system captures images of each drop to confirm monoclonality, which is critical in regulated applications.
What’s unique is that we can select cells based on morphology or fluorescence markers, use any sample volume or plate format, and achieve near-100% single-cell accuracy. The dispensing process is incredibly gentle, minimizing cell stress and maximizing viability for downstream clonal outgrowth.
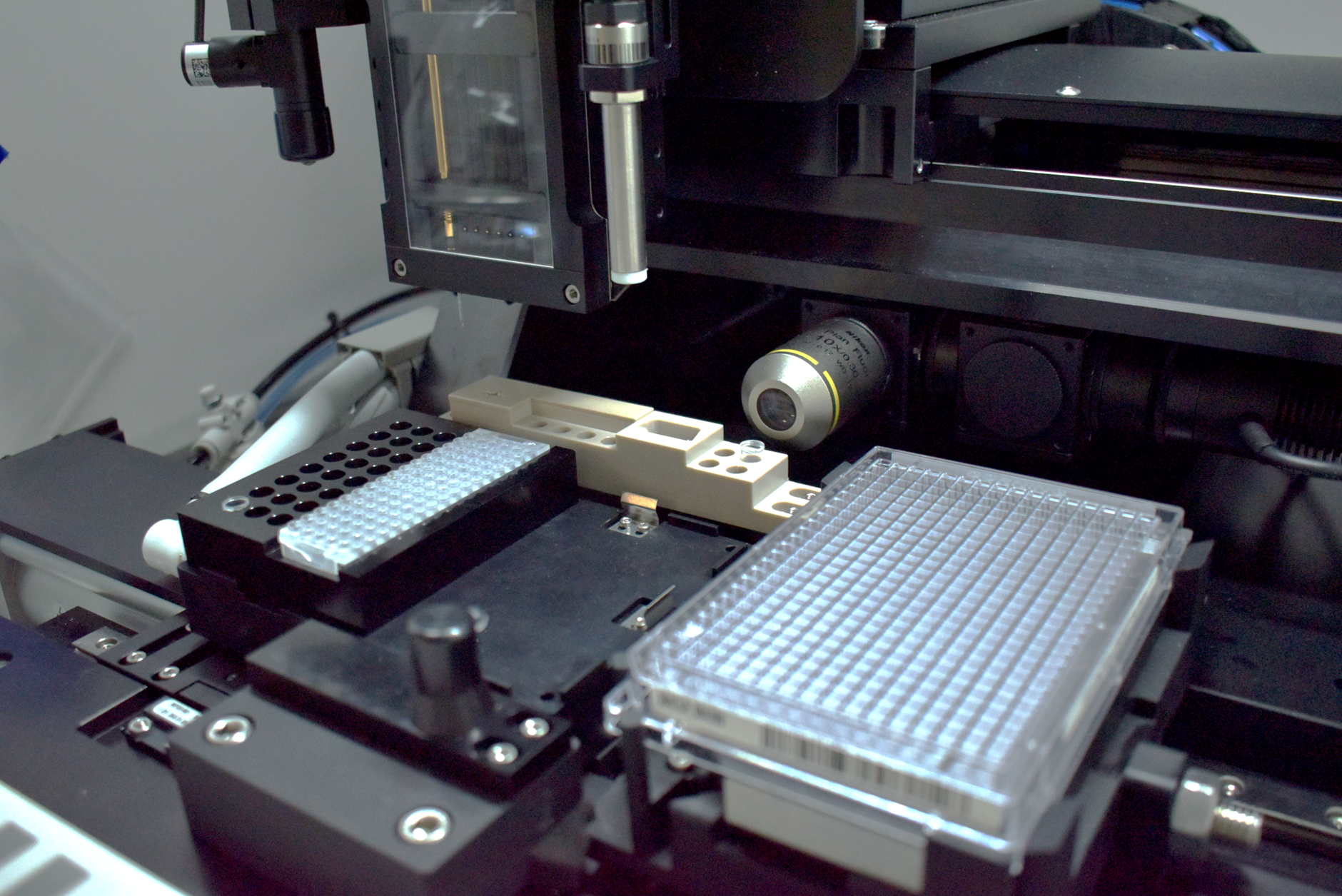
Image Credit: Cellenion
What are the key limitations of FACS that you aimed to solve with this solution?
Mathieu Bennet: The first major limitation is cost and labor. FACS systems need a skilled operator present at all times, and they use consumables that add to operating costs. There is also maintenance downtime, especially in GMP environments.
The second issue is throughput. Because FACS is manual and involves many steps, like sample loading and calibration, it is hard to scale. The high-pressure sorting can also affect cell viability. With cellenONE, we address all these issues by automating the process, using a gentle sorting mechanism, and integrating seamlessly into existing lab workflows.
How does automation play a role in enabling high-throughput B-cell cloning?
Dustin Whitson: Automation is the game-changer. We have worked with Cellenion to create a fully integrated platform using our Green Button Go (GBG) scheduling software. The system includes the cellenONE HT, an incubator, and a robotic arm that transfers plates between devices.
From sample loading to dispensing and plate handling, everything is hands-free. The entire workflow is error-tolerant, with built-in automated calibration, QC checks, and recovery methods. Our GBG software orchestrates all these components so that scientists can walk away and return to fully populated microtiter plates.
What advantages does the CellenONE bring in terms of cell viability and clonal outgrowth?
Mathieu Bennet: Our proprietary dispensing mechanism is exceptionally gentle. This is vital in B-cell cloning, where every cell matters, especially if the sample source is rare or limited. Because of how we protect the cells during isolation, we have seen the best clonal outgrowth rates on the market.
Every image of every isolated cell is recorded, ensuring full traceability. This is particularly important in GMP settings where monoclonality must be demonstrated.
Note that cellenONE can also be used for any type of single cell applications, from single cell omics (genomics, transcriptomics, proteomics, metabolomics and multi-omics) to synthetic biology, and can isolate and sort any type of cells and membrane compartment, from mitochondria and bacteria to cardiomyocytes.
Could you describe a typical workflow using the automated CellenONE HT platform?
Mathieu Bennet: The process starts with sample aspiration from any container, like a tube or well plate. The nozzle moves in front of a microscope to scan for single cells. If a suitable cell is detected, it is gently dispensed into a target well.
Empty or unwanted drops are recovered, meaning there is no wastage. This continues until the entire plate is filled. All images are saved, and once completed, the robotic arm returns the plate to the incubator. It is fully automated and requires minimal setup, an ideal solution for scaling B-cell workflows.
cellenONEHT
Video Credit: Cellenion
How does your solution integrate with third-party systems?
Dustin Whitson: We have developed a robust API that allows the cellenONE platform to be fully controlled through third-party systems like GBG. This means labs can schedule, monitor, and troubleshoot the workflow remotely.
It also supports integration with broader laboratory information systems (LIMS), so users can build complex automation strategies for B-cell cloning, antibody screening, and beyond. The platform's flexibility and modularity make it easy to adapt to different lab environments.
What is the broader vision for B-cell cloning technology at scale?
Mathieu Bennet: We believe the future of B-cell cloning lies in democratizing high-throughput, automated workflows. Our vision is to enable any lab to adopt single-cell techniques for therapeutic antibody discovery regardless of size or resources.
By making technology scalable, traceable, and user-friendly, we can accelerate innovation in immunotherapy, vaccine development, and more. The collaboration between Cellenion and Biosero is a step toward realizing that future.
About Mathieu Bennet 
Mathieu Bennet earned his PhD in physico-chemistry from the University of Edinburgh in 2011, following an MSc in Photonics from St Andrews and a BSc in Physics from Heriot-Watt University.
He joined Cellenion in 2018 and has since held multiple roles, including Senior Scientist, Engineering Manager, and Product Manager. Currently serving as Chief Technology Officer, he brings deep expertise in microfluidics, microscopy, and innovation leadership to the company’s single-cell technology development and strategic direction.
About Dustin Whitson 
Dustin Whitson holds a Master’s in Forensic Serology and DNA from the University of Florida and a Bachelor’s in Microbiology from Texas Tech University. Since 2022, he has worked at Biosero as an Applications Consulting Scientist, supporting lab automation solutions through scientific expertise and hands-on collaboration with customers.
About Cellenion
Cellenion was founded in 2016 in Lyon, France, as a spin-off company of SCIENION GmbH, which is based in Berlin, Germany.
Cellenion uses SCIENION’s high precision non-contact dispensing technologies and provides solutions for single cell analysis and cloning.
Our passionate team of scientists shares your fascination with single-cell applications and helps you move your science forward.
About BICO
BICO is a lab automation partner and provider of selected workflows to pharma and biotech. With 48,000+ instruments installed in over 65 countries, BICO products, software, and solutions are found in more than 3,500 laboratories, including the world’s top 20 pharmaceutical companies, and have been cited in over 11,900 publications. Operating through two business areas – Lab Automation and Life Science Solutions – BICO strives towards the vision to enable and automate the life science lab of the future. BICO is listed on Mid-Cap, Nasdaq Stockholm under BICO. www.bico.com