Autoantibodies (AAbs) produced by the humoral immune system represent a promising avenue for early detection, monitoring and drug development as they can be produced in response to tumor antigens several years before clinical diagnosis.1,2
AAbs target proteins that are altered during disease pathogenesis, such as abnormal expression in terms of location and level, aberrant folding, and post-translational modifications.3
Profiling AAbs aids in the identification of tumor-relevant proteins that are modified during cancer, offering important insights into the mechanisms of disease.
Type 1 MAGE proteins, for example, only display normal expression during embryonic development in the male mammalian germline. These cancer-testis antigens (CTAs), however, are expressed abnormally in non-germ cells for both men and women with cancer, triggering the generation of AAbs.4-6
AAbs as biomarkers
AAbs produced during the early stages of tumorigenesis provide a valuable opportunity for faster diagnosis. This allows for intervention prior to symptoms, a wider range of treatment options (including less aggressive treatments), and better survival rates.
For public health systems, early diagnosis could decrease the long-term burden of cancer treatment in terms of patient quality of life and treatment costs. In addition, early-stage treatments are commonly less resource-intensive, allowing for the improved distribution of therapeutic resources and access to care.
AAbs can be utilized as biomarkers to forecast patient outcomes and aid in the identification of new therapeutic targets. For example, it has been demonstrated that a signature of 13 AAbs is predictive of poor survival rates in patients with resected non-small cell lung cancer.5 This signature was validated in an independent cohort with a sensitivity of 84 % and a specificity of 74 %.
Another study identified a signature of 10 novel AAb biomarkers of melanoma with a sensitivity and specificity of 79 % and 84 %, respectively.7 The AAbs targeted transcription factors, regulatory proteins and kinases that are expressed in a variety of cells and tissues, the latter being significant as they are heavily targeted for therapeutics.
Recently, another type of post-translational modification called histone citrullination has been associated with various cancers.8,9
Further underscoring their utility, AAbs obtained from blood samples represent the full repertoire of antibodies from various tissues, as they circulate system-wide. This is especially vital as chronic diseases, such as cancer, frequently affect multiple organs and tissues. Proteins in blood, however, only represent proteins secreted or released into the bloodstream.
AAbs are suitable for retrospective and longitudinal studies due to their greater resilience against degradation compared to RNA and protein. Since AAbs stay stable for one month at room temperature and for 200 days when stored at -20 °C on dried blood spot collection cards, population screening in rural and low-income areas is achievable.10
Use of protein arrays
By employing functional protein microarrays, antibodies may be screened against hundreds to thousands of proteins simultaneously. With 90 % of humoral antibodies binding conformational epitopes, AAb screening tools should utilize correctly folded proteins that preserve those critical epitopes for accurate and meaningful results.11
Sengenics arrays leverage patented KREX® technology to guarantee proper folding of full-length proteins for highly specific antibody binding, as shown in Figure 1.12 The array surface is also modified to offer an aqueous environment for the proteins to float naturally.
Significantly, denatured, linear and improperly folded proteins on other arrays may expose “sticky” hydrophobic sequences, leading to nonspecific antibody binding and inaccurate data.
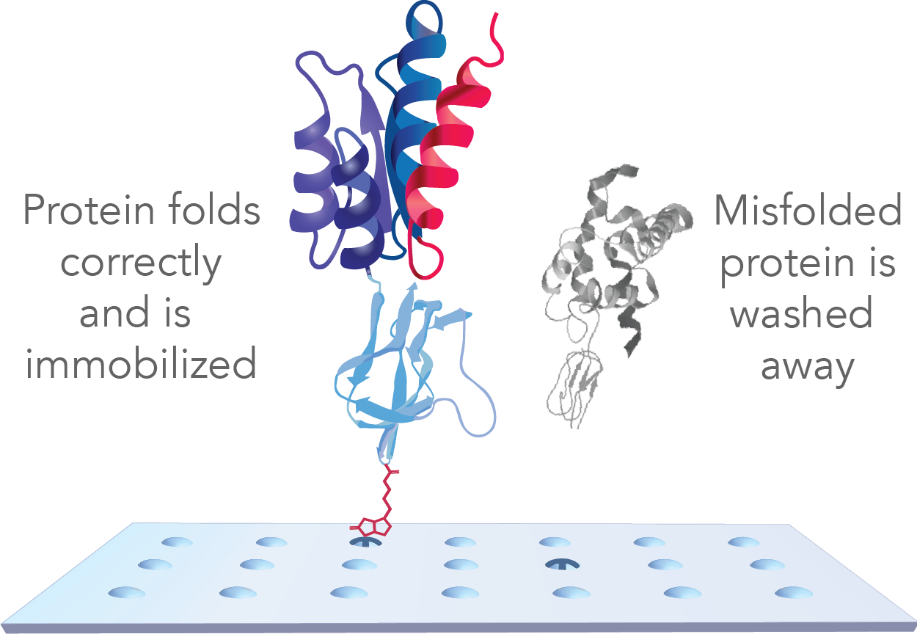
Figure 1. Schematic of a Sengenics high-density protein array for antibody profiling. With KREX technology, every protein is tagged with a biotin carboxy carrier protein (BCCP) that binds to biotin only when correctly folded. The proteins are then anchored to the array surface through a stable biotin-streptavidin bond. Misfolded proteins result in a BCCP that cannot bind to biotin, and are consequently washed away and removed from further analysis. Image Credit: Sengenics Corporation LLC
Conclusions
Despite being a genetic disease, genetic testing of cancer does not reflect the dynamic biological changes that occur in real-time. AAb profiling addresses this issue by providing a direct view into the immune response to cancer and identifying proteins with cancer-induced modifications. Furthermore, AAb data can be used alone or in conjunction with other omics datasets to translate complex disease profiles into actionable clinical insights, advancing precision medicine and improving patient outcomes.
References
- Sexauer, D., E. Gray, and P. Zaenker (2022). Tumour- associated autoantibodies as prognostic cancer biomarkers- a review. Autoimmun Rev, 21(4), pp.103041.
- Anderson, K.S. and J. Labaer. (2005) The Sentinel Within: Exploiting the Immune System for Cancer Biomarkers. Journal of Proteome Research, 4(4), pp.1123-1133.
- de Jonge, H., et al. (2021) Anti-Cancer Auto-Antibodies: Roles, Applications and Open Issues. Cancers (Basel), 13(4).
- Weon, J.L. and P.R. Potts. (2015) The MAGE protein family and cancer. Curr Opin Cell Biol, 37, pp.1-8.
- Patel, A.J., et al. (2022) A highly predictive autoantibody-based biomarker panel for prognosis in early-stage NSCLC with potential therapeutic implications. Br J Cancer, 126(2), pp.238-246.
- Zaenker, P., et al. (2018) A diagnostic autoantibody signature for primary cutaneous melanoma. Oncotarget, 9(55), pp.30539-30551.
- Wang, M., et al. (2024). Biomarkers of peripheral blood neutrophil extracellular traps in the diagnosis and progression of malignant tumors. Cancer Medicine, 13(3), pp.e6935.
- Song, S. and Y. Yu. (2019) Progression on Citrullination of Proteins in Gastrointestinal Cancers. Front Oncol, 9, pp.15.
- Tong, L., et al. (2023). Development of an autoantibody panel for early detection of lung cancer in the Chinese population. Front Med (Lausanne), 10. pp.1209747.
- Amini, F., et al. (2021) Reliability of dried blood spot (DBS) cards in antibody measurement: A systematic review. PLoS One, 16(3), pp.e0248218.
- Van Regenmortel, M.H.V. (1996) Mapping Epitope Structure and Activity: From One-Dimensional Prediction to Four-Dimensional Description of Antigenic Specificity. Methods, 9(3), pp.465-72.
- Beeton-Kempen, N., et al. (2014) Development of a novel, quantitative protein microarray platform for the multiplexed serological analysis of autoantibodies to cancer-testis antigens. Int J Cancer, 135(8), pp.1842-51.
About Sengenics Corporation LLC
 Sengenics is a leading proteomics company providing high-density protein arrays for the discovery and validation of autoantibody biomarkers. Their innovative approach leverages their patented KREX® technology to preserve crucial three-dimensional epitopes that antibodies recognize, ensuring accurate and meaningful results. Autoantibody biomarkers enable early disease detection, drug response prediction, patient stratification, and the development of companion diagnostics.
Sengenics is a leading proteomics company providing high-density protein arrays for the discovery and validation of autoantibody biomarkers. Their innovative approach leverages their patented KREX® technology to preserve crucial three-dimensional epitopes that antibodies recognize, ensuring accurate and meaningful results. Autoantibody biomarkers enable early disease detection, drug response prediction, patient stratification, and the development of companion diagnostics.
Headquartered in the USA and supported by a global network of offices, distributors, and service providers, Sengenics remains committed to advancing personalized medicine worldwide.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.