Every successful laboratory operates at the intersection of expertise and technology: the right individuals utilizing the best, most efficient tools to perform research, assess quality, ensure compliance, and meet their scientific and business objectives.
Access to a modern informatics suite is crucial for supporting the above successful formula, whether on-premise or as a cloud-hosted solution. This guide explores a third option that is gaining popularity among laboratories of all sizes—from entry-level to enterprise: software-as-a-service (SaaS).
A subscription-based Laboratory Information Management System (LIMS), hosted in the cloud and accessible via the internet, serves as a secure, scalable, and cost-effective alternative to conventional software licensing, installation, maintenance, validation, and management. Here’s what needs to be understood to determine if the software-as-a-service (SaaS) delivery model for a LIMS is suitable for an organization.
One of Mark Twain’s principles for effective writing was to be clear and concise. “Eschew surplusage,” the renowned storyteller famously stated in a 1895 critical essay. This advice remains valuable for aspiring writers – and even established authors – today. But it can also extend beyond literature. Indeed, why complicate personal and professional lives with unnecessary or non-essential matters when simplicity and a seamless experience enable achieving as much, if not more?
That philosophy underlies the rising trend to offer cloud-hosted, software-as-a-service (SaaS) delivery of applications, including many relied on daily. Consider Google Apps, Microsoft Office 365, Dropbox, and Slack, to name just a few prominent SaaS brands.
The laboratory informatics sector is also shifting toward cloud – not just for data storage or hosting a purchased LIMS solution. There’s a readiness to abandon traditional models of licensing, installation, maintenance, and management software in favor of engaging with a web-based service provider that delivers a right-sized application and simultaneously assumes responsibility for back-end security, availability, and performance.
Traditional challenges
To comprehend the increasing appeal of a SaaS LIMS, one must first understand why some organizations prefer to maintain their existing implementations. For those that acquire software to meet their specific needs and are supported by robust IT departments, an on-premise LIMS has compelling advantages.
Organizations that are particularly risk-averse may wish to retain more control over their data by keeping it in-house. The need for a stable, predictable LIMS, perhaps alongside components like Electronic Lab Notebook (ELN), Lab Execution System (LES), and Scientific Data Management System (SDMS), outweighs other factors, such as slower upgrade cycles and the risk of service interruptions during hardware or software maintenance.
However, these reasons highlight the challenges inherent in purchasing a LIMS. There could be significant up-front costs associated with a perpetual license, which might not concern a large global enterprise but could be prohibitive for many smaller companies and laboratories with tighter budgets. Hardware, sufficient IT staffing, and the expenses linked to maintaining a secure data center require substantial investment, as well.
A managed cloud solution can alleviate many of these concerns for organizations of any size. In an Infrastructure-as-a-Service (IaaS) model, the LIMS software is purchased, but installation and hosting occur on virtual servers at remote facilities, either for a fee or through a subscription-based arrangement. The customer accesses the LIMS via a secure connection and a web browser, without being involved in infrastructure management. Service level agreements provide additional assurance regarding availability; however, the customer remains responsible for software administration and maintenance.
In this context, it is clear why a SaaS-based LIMS might be an appealing third option for many organizations.
A web-based alternative
With a SaaS model, companies can access an advanced informatics suite without a substantial upfront capital investment in licensing and infrastructure or paying for unnecessary features. They are essentially renting access to cloud-hosted applications and the features they require, along with storage for their LIMS and additional data.
Servers, networking, load balancing, security, auto-failover, and backups are included in the subscription package, and because the vendor manages software updates, users always have access to the latest version via their preferred web browser – whether they are in the same lab, a different building, or another part of the world. There’s no additional installation or validation needed on a workstation, laptop, or mobile device.
Access to specific features can be toggled on or off – a characteristic common across SaaS applications designed for personal and professional use. Subscribers might select from various plans, such as Standard, Advanced, and Enhanced, each with different pricing based on their need to support a defined number of users and/or access increased storage, load balancing, failover and disaster recovery, testing parameters, or other features. This flexibility can adapt to an organization’s evolving requirements as well. Indeed, instant scalability to meet business demands is a core advantage of a subscription-based model.
Additionally, a vendor can configure its SaaS offering as pre-packaged, industry-specific solutions addressing unique workflows – in biobanking, cancer research, contract testing, diagnostics, healthcare, and quality assurance, or the food and beverage and oil and gas sectors, for instance – which further tailors a LIMS for an organization, eliminates the need for customizations, accelerates adoption and deployment, and reduces costs. For LIMS customers in regulated industries, vendor validation of the SaaS environment is crucial.
Benefits of SaaS validation
Pharmaceutical, healthcare, food and beverage, and similar industries can now leverage validated SaaS, where the vendor ensures the delivered system is managed and maintained in a controlled, risk-aware manner. The vendor takes responsibility for changes made to the delivered software during feature enhancements, software upgrades, and updates made to the LIMS, ensuring that every alteration is fully documented, tested, and reported to the user. This evidence of validated control is essential when a company must report to the FDA or similar regulatory body.
Such vendor support guarantees that the system meets the highest standards for data integrity and system control, with the responsibility for maintaining those standards resting with the vendor.
Validated SaaS is vital in a large or growing organization because it allows the LIMS to be deployed confidently and even expanded to other locations, subsidiaries, and partners.
Common scenarios
Pricing is one of the foremost concerns when a laboratory evaluates its options for a LIMS. According to a recent study by LabVantage, considering that most LIMS remain in use for roughly seven years before they are significantly upgraded or replaced, along with the average annual costs linked to hardware and IT resources, a SaaS-based LIMS could save as much as 32 percent compared to the costs associated with an on-premise solution.
However, budget alone should not dictate the decision. One of the first questions a decision-maker should ask is: “What’s needed?” Followed by: “And why?”
This leads to several scenarios where a SaaS-based LIMS may be most appropriate.
- For organizations that have already embraced the cloud and are technologically adept, or where leadership has chosen to adopt a cloud-first strategy, the answer to “why” could take precedence. Others lacking robust IT and financial resources might find a cloud-based solution a natural fit for their needs.
- Laboratories of all sizes with simple or common routines that don’t require a fully featured or configured informatics suite represent another category well-suited to the SaaS model.
- Mergers and acquisitions, contract or offshore projects, and other situations where a solution may be needed temporarily or during transitions may benefit from the built-in security, scalability, flexibility, and ease of adoption that a subscription LIMS provides.
On-premise, managed cloud, or SaaS solution?
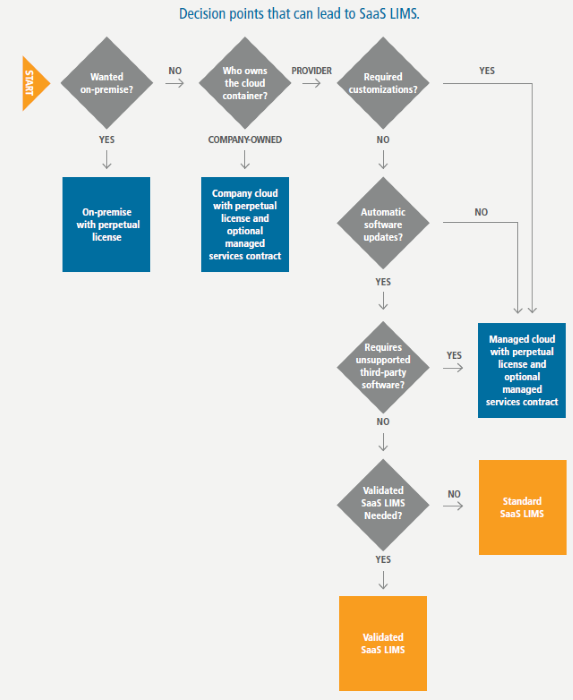
Image Credit: LabVantage Solutions
The path to SaaS
Choosing between an on-premise, managed cloud, or SaaS solution – validated or not – involves numerous decision points. One way to assess the decision-making process is through a decision tree.
An organization that wishes to maintain its LIMS on-premise or customize one cannot move to SaaS. If purchasing a perpetual license is preferred, the only options are an on-premise installation or a cloud-hosted solution with or without a managed services contract.
However, for those who seek a less burdensome approach and value effortless and cost-effective access to an always-up-to-date laboratory informatics suite with fully integrated LIMS, ELN, LES, and SDMS, SaaS represents a viable alternative. With vendor validation, it’s available for regulated industries.
Laboratories of all sizes—from entry-level to enterprise—can benefit from shedding the expensive overhead associated with traditional systems and paying only for what they need and use via SaaS-based LIMS.
References
- LabVantage. (2020). LIMS Total Cost of Ownership: Cloud Hosting versus On-Premises - LabVantage. (online) Available at: https://www.labvantage.com/lims-total-cost-of-ownership-cloud-hosting-versus-on-premises/ (Accessed 18 Jul. 2025).
About LabVantage Solutions
LabVantage Solutions, Inc. is the leading global laboratory informatics provider. Our industry-leading LIMS and ELN solution and world-class services are the result of 35+ years of experience in laboratory informatics. LabVantage offers a comprehensive portfolio of products and services that enable companies to innovate faster in the R&D cycle, improve manufactured product quality, achieve accurate recordkeeping and comply with regulatory requirements.
LabVantage is a highly configurable, web-based LIMS/ELN that powers hundreds of laboratories globally, large and small. Built on a platform that is widely recognized as the best in the industry, LabVantage can support hundreds of concurrent users as well as interface with instruments and other enterprise systems. It is the best choice for industries ranging from pharmaceuticals and consumer goods to molecular diagnostics and bio banking. LabVantage domain experts advise customers on best practices and maximize their ROIs by optimizing LIMS implementation with a rapid and successful deployment.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.