Sponsored Content by LabmanReviewed by Maria OsipovaNov 3 2025
Labman developed a point-of-care diagnostic instrument engineered for HPV screening through the successful operation of a highly complex microfluidic ‘lab-on-a-chip’.
The EU-funded ELEVATE project aimed to design and validate a cervical cancer screening instrument capable of providing rapid, easy-to-interpret results at a low cost. Alongside a multinational team of partners who conducted the research, Labman’s role as the integration technical partner was to develop an instrument capable of reliably actuating a sophisticated microfluidic cartridge.
Despite numerous challenges, the completed device consistently executes the assays through precise control of liquids on a microfluidic scale. With its portability, support for patient self-sampling, and rapid result reading, the device is expected to significantly improve access to healthcare for thousands of women in hard-to-reach communities around the world.
Preventative screening for hard-to-reach populations.
Cervical cancer ranks as the fourth most common cancer among women, with hard-to-reach populations in various regions facing an increased risk of developing the disease. Preventive screening programs and vaccination against human papillomavirus (HPV) have been shown to reduce cancer incidence and mortality.
The overall goal of the project was to improve cervical cancer screening coverage, especially for women who are geographically or culturally isolated. The point-of-care detection device is engineered to identify HPV infections and biomarkers indicating cancer development in women from diverse ethno-geographic backgrounds and socioeconomic groups.
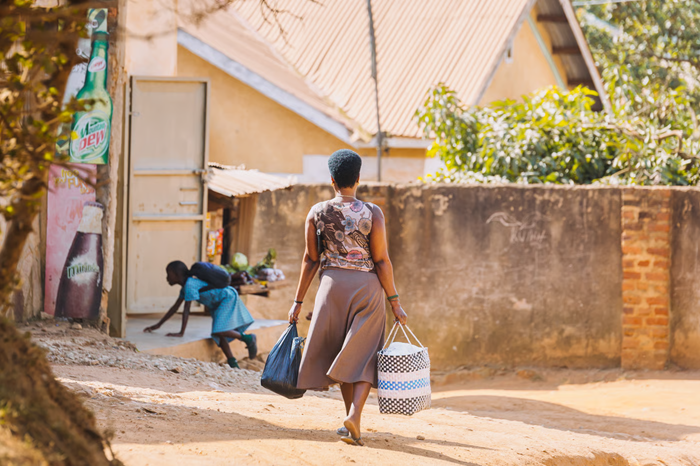
Image Credit: Labman
Compact and portable
The solution is a portable, point-of-care HPV testing device that delivers quick, easy-to-interpret results without requiring trained medical personnel. The device is capable of fully automated detection of 14 HPV strains and two proteomic biomarkers, with the only end-user intervention being their introduction of the self-sample swab. Capable of obtaining results ‘while-you-wait’, the device delivers quantitative data for each HPV strain and the proteins.
Compatible with self-sampling
For many women around the world, cervical cancer screening remains a time-consuming and stressful process requiring a trip to a medical facility and a gynecological examination, typically involving a cervical smear. This approach also demands trained personnel and laboratory equipment, which are frequently unavailable in resource-limited regions.
A more accessible test based on self-sampling could improve cervical cancer screening rates among women in isolated populations or low-income countries, as well as among women who, for various reasons, do not engage with screening programs.
Comprehensive virus detection
HPV DNA tests used for screening strategies are limited by their low specificity in identifying transforming infections with a high potential to drive pre-cancer and cancer. This limitation might be even more significant when testing women from diverse ethno-geographic backgrounds, as certain HPV variants are associated with specific patient origins. The device sequences DNA from 14 high risk HPV types and verifies the genomic variation.
Identifying cancer progression
The concurrent detection of HPV DNA with two proteomic biomarkers enables more precise identification of HPV infections linked to cervical cancer progression. Positive cases for both genomic and proteomic tests are classified as high-risk cases for cancer development, enabling any patients who test positive to be referred for additional examination and potential treatment.
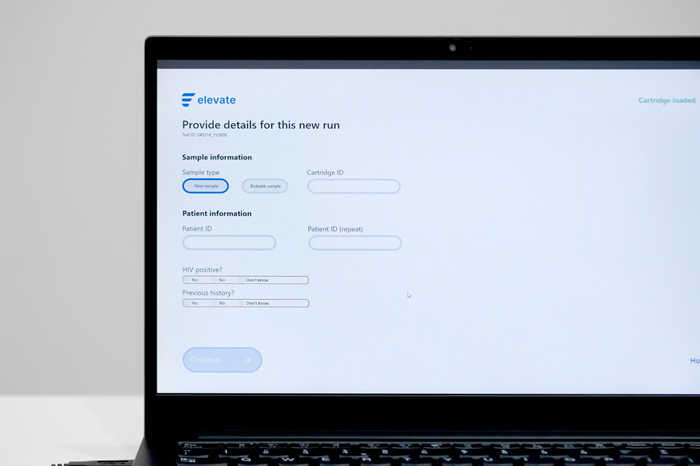
Image Credit: Labman
The challenge of microfluidics
Historically, microfluidics has presented multiple challenges in biomedical applications, especially in diagnostic instruments, where minor interactions can lead to inaccurate assessments.
ELEVATE consortium partners at the University of Rovira i Virgili and the University of Ghent conceptualized and developed a novel assay enabling the combined detection of 14 oncogenic HPV DNA types and the expression of two cervical cancer protein biomarkers. This assay involves multiple stages comprising complex biochemical reactions.
Consortium partner Fraunhofer IMM translated these assay stages into a complex microfluidic network consisting of sample collection ports, microfluidic channels, blisters and capsules containing sensitive freeze-dried and liquid reagents, gold-electrode arrays, and waste collection chambers.
The microfluidic network design was subsequently optimized for high-volume production by another consortium partner at microLIQUID (now TE Connectivity). The entire cartridge is no larger than the palm of your hand.
Handling, positioning, and accurate flushing of numerous liquids inside the cartridge’s channels was extremely challenging. Rainer Gransee of IMM collaborated with a team of biologists and engineers to design the cartridge.
It was only possible through the implementation of Labman’s sophisticated optical plug flow detection using an integrated camera in combination with clever software for precisely controlling pumps and valves. This allowed the subsequent flushing, mixing, incubation, washing and the final read-out of the analytes to be detected.
Rainer Gransee, IMM Fraunhofer

Image Credit: Labman
Groundbreaking results
ICREA Research Professor Ciara O’Sullivan, an expert with three decades of experience, established the Nanobiotechnology and Bioanalysis Group at the Universitat Rovira i Virgili and contributed to the assay’s development. Ciara spoke about the high degree of precision required for the microsystem and instrumentation to function together in order to successfully complete the assay.
Microsystems, they all have the same objective, sample in, result out. I’ve never seen any single system work. Most of the time, either you get the fluids just flying through the system and there’s no control, so there’s no proper mixing or measurement. Or, the liquid stops halfway through because there isn’t enough force to propel the liquid through the microsystem. The success is totally dependent on the design of the microsystem and the design of the instrumentation.
What Labman have done, they’ve developed this instrumentation that truly makes everything automated – I’ve seen it again and again, it literally is sample in, result out. I’ve worked together with many, many companies over the years and while there has been some successes, no one has been able to achieve this.
The detection part of the technology is quite complex, its electrochemical detection. Labman have developed this really fantastic potentiostat that is able to measure 64 different markers within six seconds. That doesn’t exist. There is no other potentiostat on the market that can do that. It’s more than impressive.
Ciara O'Sullivan, ICREA Research Professor

Image Credit: Labman
Next steps for ELEVATE?
85 % of women who die from cervical cancer, an entirely preventable disease, live in developing nations. ELEVATE is undergoing clinical trials to evaluate the technology using real patient samples and to confirm the results using current laboratory techniques.
These promising advancements could significantly improve HPV infection detection, strengthen healthcare systems, ultimately saving the lives of thousands of women. The successful creation of the device stands as a testament to the exceptional collaboration between multinational partners.

Image Credit: Labman
Collaboration within the consortium was amazing, especially with all technical partners, taking into account the highly specialized expertise of each partner. These reach from electrochemistry, to microfluidics, to mechanical design, automation and manufacturing knowhow making it necessary to find the right terms and to think alike with the project success in mind. There was a huge commitment being necessary, especially shown by Labman responsible for 'knitting' all the parts together in the end.
Rainer Gransee, IMM Fraunhofer

Image Credit: Labman
Acknowledgements
Produced from materials originally authored by Katie Simpson, Content Lead at Labman.
 About Labman
About Labman
Labman is a global leader in creating custom automated laboratory technologies, products, and software that empower and advance scientific discovery. We collaborate across diverse industries, including medical, pharmaceutical, agritech, paints and coatings, and FMCG, to deliver innovative solutions. From our state-of-the-art manufacturing facilities, Labman’s multi-disciplinary teams design, build, and support systems used worldwide, enabling groundbreaking science in leading laboratories every day.
Labman in 2025
Video Credit: Labman
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.