Knowing a drug’s bioavailability during lead optimization is essential as it directly influences dosing strategy, safety margins, therapeutic efficacy, and formulation design.
Historically, bioavailability estimates have been dependent on animal studies, which often fail to predict human in vivo outcomes accurately while simultaneously incurring significant costs. Traditional approaches also rely on isolated in vitro assays that often fail to accurately predict human drug absorption and metabolism.
These issues can lead to potentially expensive late-stage failures, with Waring et al. (2015) reporting that pharmacokinetics and bioavailability are the third most common cause of attrition (16 % of failures) in Phase I clinical trials for compounds developed by large pharmaceutical companies.
This article looks at how CN Bio reduces reliance on animal use by integrating in silico tools with a fluidically interconnected gut-on-a-chip and liver-on-a-chip to deliver more accurate bioavailability predictions.
The PhysioMimix® advantage
CN Bio adopted a novel approach for addressing these limitations using a combination of primary human gut and liver microphysiological systems (MPS, also known as gut-on-a-chip or liver-on-a-chip) coupled with advanced computational modeling. This powerful integrated strategy bridges the gap between in vitro testing and in vivo outcomes, delivering a more human-relevant alternative able to:
- Enhance prediction accuracy
- Lower reliance on animal studies
- Provide a more cost- and time-efficient process
CN Bio’s PhysioMimix system enables the study of intestinal absorption and hepatic clearance in a single, interconnected system. This approach is distinct from traditional methods, which assess these functions in isolation. PhysioMimix’s connectivity is designed to mimic human physiology, giving users a holistic understanding of bioavailability.
Researchers often express concern about whether MPS platforms offer proven advantages over conventional models and how complicated it might be to transition from familiar assays to organ-on-a-chip systems.
Image Credit: CN Bio
CN Bio has a proven track record of supporting its customers in managing this transition. Customers can either gain rapid access by outsourcing testing to its Contract Research Services or bring the model in-house with support from their Field Application Scientist Team.
Regulatory shifts, including the FDA Modernization Act 2.0 and 5-year roadmap to phase out animal testing requirements for monoclonal antibodies (with plans to extend these changes to other drug classes), plus the UK announcement to phase-out animal testing faster, which specifically cites reductions in dog and non-human primate pharmacokinetic studies by 2030, continue to accelerate the adoption of New Approach Methodologies (NAMs).
Enhancing MPS with computational modeling
The integration of in silico tools with organ-on-a-chip workflows improves the interpretation of results and allows the use of MPS-derived parameters in downstream physiologically based pharmacokinetic (PBPK) modeling.
The FDA and UK government has stressed the increasing importance of in silico tools in their roadmaps to reducing animal testing in preclinical safety studies, emphasizing the value of integrating simulation tools and experimental data.
The importance of mechanistic modelling
In addition to using in silico tools to enable downstream physiologically based pharmacokinetic (PBPK) modeling of MPS-derived parameters, CN Bio is developing, and plans to commercialize, computational models to facilitate advanced in vitro assay adoption. These models will enable in silico simulation of experiments before reaching the wet lab to eliminate guesswork in setting drug concentrations and time points for sampling.
This predictive power facilitates optimized experimental design and allows for refinement of sampling times, dosing concentrations, and other key variables, enabling experimentalists to generate meaningful results more efficiently.
The PhysioMimix Bioavailability assay, along with computational tools, guides every step, eliminating the guesswork involved in setting drug concentrations and sampling time points.
CN Bio’s computational models are based on the mechanistic details underlying the gut/liver-MPS, enabling the extraction of meaningful ADME parameters from complex datasets.
Many of these parameters would be difficult to quantify using conventional approaches, meaning they can now be used to better inform PBPK modeling or in vitro to in vivo extrapolation (IVIVE).
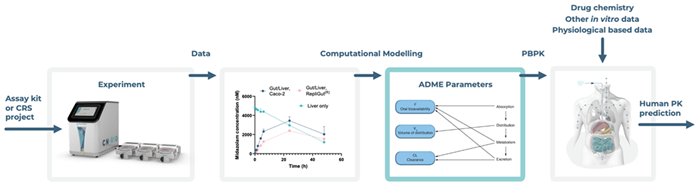
Image Credit: CN Bio Innovations Ltd
Each replicate of an MPS experiment can be used to identify multiple pharmacokinetic parameters that would historically require separate assays to determine. Mechanistic modeling is also key to gaining potentially deeper biological insights, including critical questions such as: “At what concentration does the system show non-linear behavior?” or “When does saturation occur?”
Integrating in silico tools with organ-on-a-chip technology allows these questions to be answered with greater precision at a significantly reduced cost versus the equivalent animal studies.
Integrating in silico tools with organ-on-a-chip: A midazolam case study
A peer-reviewed publication by Abbas et al. (2025) highlights a novel workflow using organ-on-a-chip data to determine pharmacokinetic parameters and the bioavailability of midazolam.
A single set of experiments made it possible to quantify the midazolam concentration over 72 hours, with data revealing that the compounds were being absorbed and translocated through the gut membrane before entering the liver compartment, where the liver metabolized the majority of the compound.
Mathematical models were developed to describe the movement of midazolam throughout the MPS. This process allowed several feasible models to be generated, each with distinct assumptions, which were all fitted to the experimental dataset.
Next, each model was ranked according to a performance criterion before selecting the best-performing model. They used Bayesian methods to determine the confidence intervals of key parameters such as intrinsic hepatic and gut clearance (CLint, liver and CLint, gut), efflux ratio (Er), and apparent permeability (Papp).
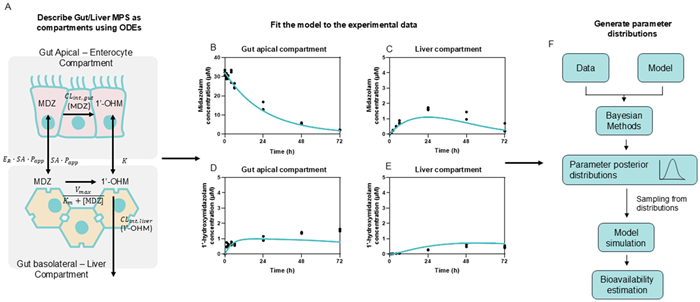
Image Credit: CN Bio Innovations Ltd
Using the model's output parameters, it was then possible to generate values for the bioavailability components Fa, Fg, and Fh (the fraction absorbed and escaping the gut and liver metabolism, respectively).
Determining the product of these three components allowed the researchers to estimate a value for the oral bioavailability of midazolam. This prediction fell within the observed range of clinical values.
Future outlook
CN Bio’s method of estimating key ADME parameters allows for organ-on-a-chip experiments to be used in the PBPK modelling pipeline. The resulting parameters can be plugged into these more complex models to potentially inform first-in-human trials.
Traditional approaches to acquiring the parameters required for PBPK modeling can be inaccurate, time-consuming, and expensive, particularly when employing animal models.
Using a combination of more relevant MPS-based experiments and computational modeling offers a less costly, more translatable method to determine a drug’s key pharmacokinetic parameters. This method also further reduces the need for animal studies in lead optimization.
CN Bio is continuing to develop its integration of in silico tools with organ-on-a-chip. The company is dedicated to further validating this method to evidence the suitability of primary MPS-based assays for lead optimization, solidifying its position as a more useful alternative to historical methods.
Summary
There continues to be hesitancy about adopting organ-on-a-chip technology into workflows due to the need for relevant internal expertise to perform experiments, proof of its effectiveness, and experimental costs. Regulatory change is on the horizon, however, which means it is the ideal time to begin integrating MPS technology.
CN Bio has simplified the onboarding journey by developing the protocols to recreate its dual-organ MPS and bioavailability assay in any suitable laboratory, plus offering the ability to bypass onboarding requirements via its ADME Contract Research Services.
The company has also developed and seeks to commercialize a range of in silico tools currently accessible to CN Bio’s ADME Contract Research Services. The company’s simulation software is designed to help plan MPS-based experiments, streamline workflows, and lower experimental costs, whilst an additional tool provides the ability to improve the quality of input parameters for PBPK modeling over traditional approaches and more accurately estimate orally administered drugs’ in vivo pharmacokinetic parameters.
Abbas et al. (2025) recently published a comprehensive summary of this workflow in Drug Metabolism and Disposition.
Rapidly integrating MPS into a workflow
CN Bio offers a range of options for organizations looking to modernize internal workflows. The company’s industry-proven PhysioMimix Core technology is available for in-house adoption, alongside ADME Contract Research Services, which sees CN Bio’s team complete experiments on its own premises.
These services can validate MPS for a specific context prior to investing in PhysioMimix technology in-house. Organizations can also outsource their project work to CN Bio’s team of experts to instantly benefit from MPS insights.
Acknowledgments
Produced from materials originally authored by CN Bio Innovations Limited.
About CN Bio
CN Bio is a leading organ-on-a-chip (OOC) company that offers a portfolio of products and contract research services to optimize the accuracy and efficiency of bringing new medicines to market. With more than a decade of research and development experience, they aim to transform the way human-relevant pre-clinical data is generated through the development of advanced in vitro human organ models.
CN-Bio's PhysioMimix® Core microphysiological system (MPS) enables researchers to recreate human biology in the lab and is the only microphysiological system with validated performance across single-, multi-organ, and higher throughput configurations. This easy to adopt, adapt and scale technology bridges the gap between traditional cell culture and human studies, to support the development of safer and more efficacious therapeutics, whilst reducing the dependence on animal model usage.
CN Bio’s portfolio of products (MPS, 3D validated cells, consumable plates) and services support researchers that require reliable, data-rich, in vitro studies, to uncover novel mechanistic insights into drug or disease mechanism of action.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.