The FDA recently announced its plan to phase out the animal testing requirement for monoclonal antibodies (mAbs) and other drugs, instead opting to use human-relevant new approach methodologies (NAMs).
This article looks at CN Bio’s PhysioMimix® Core system.
Microphysiological systems help address the shortcomings of animal models for mAb development, and CN Bio is taking an active role in supporting organizations in switching to an NAMs-based approach.
Address animal shortcomings during development using microphysiological systems for mAbs
CN Bio has developed, characterized, validated, and commercialized a diverse array of organ models and assays. These models and assays have been successfully adopted by 16 of the top 20 pharmaceutical companies, with widespread use testing a range of drug modalities across safety, toxicology, disease modeling, and ADME applications.
A validated protocol for CN Bio’s fully immunocompetent assay is not widely available at present, but its concept is verified, and its development approach is built upon proven results. Customers may, however, develop their own assay by adapting CN Bio’s current human PhysioMimix Core DILI assay in the interim.
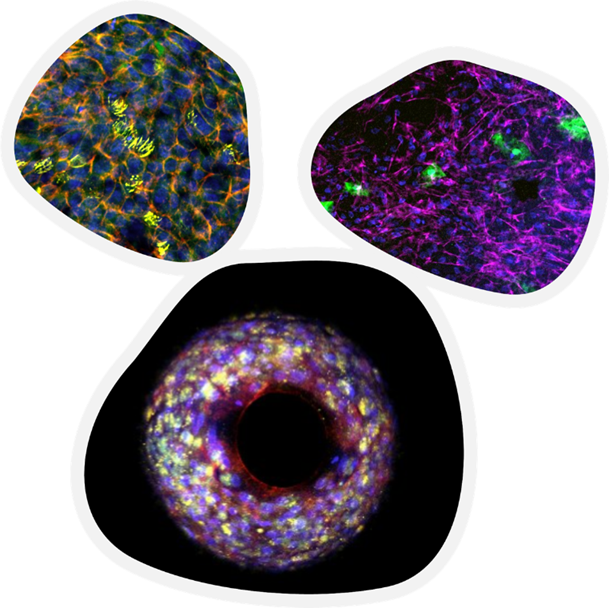
Image Credit: CN Bio Innovations Ltd
The FDA’s roadmap to reducing animal testing in preclinical safety studies focuses on immune-related risk and how microphysiological systems can add a “crucial safety net that animal tests struggle to provide.”
The use of primary human cells in microphysiological systems helps to avoid interspecies differences, revealing immune-related toxicological effects that are more relevant to humans. However, modeling the human immune system in all experimental setups, not just when working with animals, presents a number of challenges.
Microphysiological systems equipped with closed-loop microfluidic designs that can recirculate media around cultures are among the best methods for mAb immunogenicity testing.
CN Bio’s PhysioMimix system is the most advanced system in its field regarding immunocompetency.
One of CN Bio’s customers has already completed the initial characterization of a fully immunocompetent drug-induced liver injury (DILI) model, including circulating peripheral immune cells alongside liver microtissues comprised of hepatocytes and Kupffer cells.
This finding was reported at the Society of Toxicology conference, successfully showcasing the capabilities of the PhysioMimix technology.
It is anticipated that the development of fully immunocompetent microphysiological systems for mAbs will be completed soon, affording organizations and researchers the revolutionary ability to report inflammatory responses and resulting organ damage to drugs.
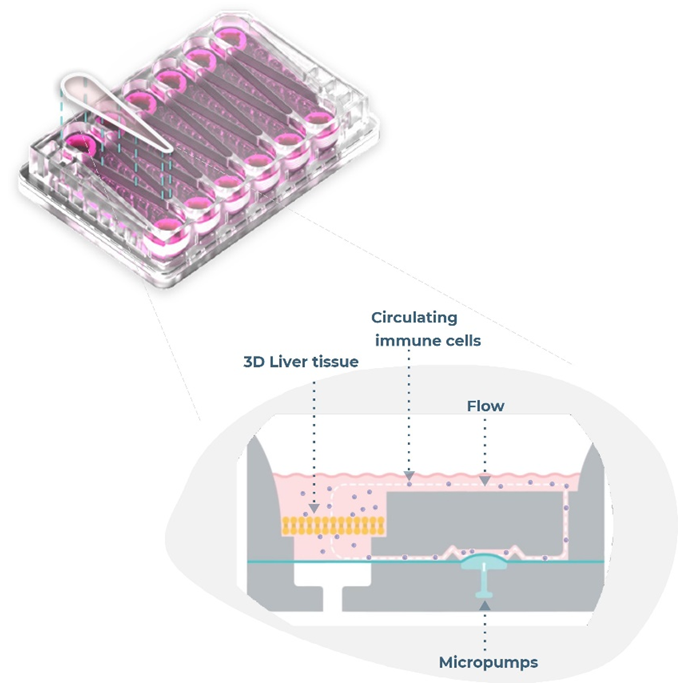
Image Credit: CN Bio Innovations Ltd
Companies, including NAMs-based data in therapeutic submissions to the FDA, will be immediately rewarded; for instance, toxicology studies using animal and NAMs methods can be reduced from six months to three, where no concerning signals are reported in the first month.
CN Bio is confident in its ability to supply effective microphysiological system-based assays suitable for these tests, allowing organizations to benefit from the FDA’s promised regulatory relief.
CN Bio has closely forged links to several primary human cell suppliers, including LifeNet Health and Lonza. These links position the company to address current market challenges related to immunogenicity testing, including HLA donor matching of primary cell types.
Its latest partnership with CRO giant Pharmaron affords CN Bio increased potential to accommodate market needs for microphysiological systems around mAbs testing even more rapidly.
Immune-mediated liver injury associated with mAbs
Four types of immune-mediated injuries are associated with monoclonal antibodies.
Idiosyncratic reactions
These reactions are unpredictable and typically not dose-related, with patients potentially developing DILI even at low drug doses.
Autoimmune hepatitis
There is a risk of mAbs triggering an immune response that causes liver damage.
Other immune-mediated injury
The primary effect of the mAb can potentially trigger an immune or non-immune response, resulting in liver injury.
Viral reactivation
Immunosuppressive mAbs have the potential to reactivate latent viral infections, which can cause liver damage, for example, HBV.
There is also an increased risk of DILI when using concomitant medications, like amino salicylates or methotrexate, with mAbs. The presence of underlying liver disease and genetic predisposition can also trigger this.
Using microphysiological systems for mAbs testing can help reduce this risk.
Liver MPS’ potential to detect immune-mediated liver injury
Human liver microphysiologcal systems are co-cultures of hepatocytes with non-parenchymal cells that are grown on scaffolds under perfusion. Cultured microtissues deliver high human liver metabolic competency and can recapitulate human-specific responses.
CN Bio’s PhysioMimix has been extensively validated for predicting small molecules’ DILI effects. Proof of concept work evaluating their use for therapeutic antibody/small molecule drug-drug interaction responses has already been demonstrated, alongside the testing of newer, more human-specific drug modalities where animal use is less suited.
There are several additional benefits to a fully immunocompetent liver microphysiological system, which incorporates peripheral immune cells into the system’s fluidic flow to circulate around liver microtissues.
Assays that use these models enable the detection of T-cell activation, cytokine release, or other immunotoxicity, such as hepatocyte damage via clinical liver function test (LFT) markers. They also enable the detection of effects that only manifest in human tissue, addressing key limitations in animal testing.
CN Bio’s fully immunocompetent assay is not widely available at present, but its concept is verified, and its development approach is built upon proven results.
A co-publication between CN Bio and the FDA’s CDER group determined that PhysioMimix systems enhanced performance versus standard techniques, and that data derived from Liver microphysiological systems is appropriate for drug safety and metabolism applications.
These results verified CN Bio’s position as a leader in the organ-on-a-chip (OOC) field, with the company’s reliable and robust cutting-edge technology determined to be ready for widespread adoption throughout the pharmaceutical industry (Rubino et al., 2021).
It is also possible to induce healthy human models to common liver diseases, such as metabolic dysfunction-associated steatohepatitis (MASH), to explore increased DILI susceptibility resulting from the presence of underlying disease.
The PhysioMimix MASH assay and kit offer proven quality, having supported Inipharm’s INI-822, a treatment for metabolic liver disease now in its clinical testing phase.
CN Bio’s role in supporting the transition away from animal testing
CN Bio continues to be actively involved in a wide range of groups, consortia, and networks that are driving change to facilitate more widespread adoption of microphysiological systems within the pharmaceutical industry.
For example, CN Bio directly and indirectly works with agencies such as ICCVAM, FNIH, and C-PATH, as well as with the FDA, to facilitate interagency coordination and accelerate the drug development process. This work involves pooling expertise, resources, and data across the US government and a range of other relevant agencies, including:
- CEN-CENNELEC, as an OOC working group
- The Predictive Safety Testing Consortium (C-PATH), as qualification workshops
- FNIH VQN (validation qualification network), as a Complement-AIRE program
- 3RsC MPS Working Group and 3RsC-FDA DILI Project aiming to build confidence in Liver MPS for DILI and regulatory applications
- NICEATM (National Toxicology Program Interagency Centre for Evaluation of Alternative Toxicological Methods) ICCVAM (Interagency Coordinating Committee on the Validation of Alternative Methods)
- NC3Rs NAMs network
- CAMs (Cambridge Alliance on Medicines) network
CN Bio’s success in supporting the transition away from animal testing towards more cost-effective and predictive workflows is rooted in its customers’ success, prompting the company to offer first-class customer support, whether customers opt to use CN Bio’s validated models and assays or develop their own novel approaches via the company’s adaptable platform.
CN Bio’s product portfolio enables the rapid and straightforward onboarding of customers with a microphysiological system-based approach. The company is also committed to broadening the system’s scope, leveraging its internal R&D efforts and strategic partnerships to enable future model and assay development.
Its inclusion in regulatory and standardization agencies and related projects means CN Bio will continue innovating and validating models for the most urgently needed applications in the drug discovery and development pipeline.
Summary
Like the FDA, CN Bio believes that the integration of more predictive NAMs methods into early decision-making is key to supporting organizations to begin reducing non-clinical animal use costs (up to $7.2M in NHP savings alone for mAb development) and making more informed go/no-go decisions on which therapeutics to advance.
Acknowledgments
Produced from materials originally authored by CN Bio Innovations Limited.
About CN Bio
CN Bio is a leading organ-on-a-chip (OOC) company that offers a portfolio of products and contract research services to optimize the accuracy and efficiency of bringing new medicines to market. With more than a decade of research and development experience, they aim to transform the way human-relevant pre-clinical data is generated through the development of advanced in vitro human organ models.
CN-Bio's PhysioMimix® Core microphysiological system (MPS) enables researchers to recreate human biology in the lab and is the only microphysiological system with validated performance across single-, multi-organ, and higher throughput configurations. This easy to adopt, adapt and scale technology bridges the gap between traditional cell culture and human studies, to support the development of safer and more efficacious therapeutics, whilst reducing the dependence on animal model usage.
CN Bio’s portfolio of products (MPS, 3D validated cells, consumable plates) and services support researchers that require reliable, data-rich, in vitro studies, to uncover novel mechanistic insights into drug or disease mechanism of action.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.