LabVantage Pharma is the only pre-configured and pre-validated pharmaceutical LIMS in the world. This greatly lowers the deployment cost (85%) and time (75%) compared to a traditional LIMS application, while offering — out of the box — the functionality and workflows needed by biotech and pharma labs. This would include batch management, consumables management, stability testing, barcode label printing, environmental monitoring, etc.
Several years of experience applying LIMS in the pharmaceutical sector form the foundation for LabVantage Pharma.
LabVantage Pharma is the perfect solution for labs having:
- A preference for fast, reduced-risk deployment
- A desire to completely leverage GAMP 5 industry best practices
- An instant need for operational, completely functional, validated LIMS
- Inadequate resources to handle a traditional LIMS implementation
- A tight budget that necessitates low total cost of ownership (TCO)
Solution Speeds Execution and Greatly Lowers Costs
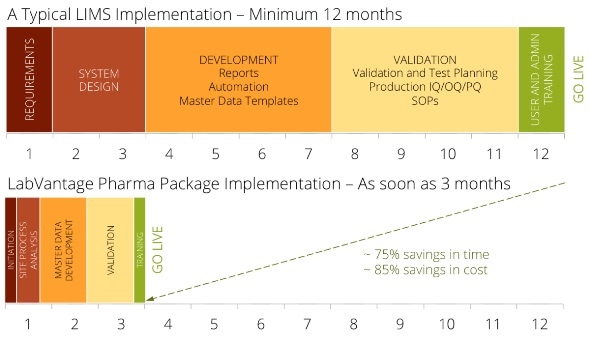
Why spend a year or longer executing a traditional LIMS — with most of that time customizing, configuring, and validating the system—when users can install LabVantage Pharma in a much lesser time?
Users can start using LabVantage Pharma within 90 days. LabVantage Solutions has made the deployment process smoother, faster, and less risky with a better, pre-validated, pre-configured LIMS, built especially for biotech and pharma labs. Designed on the foundation of LabVantage 8, the newest LIMS available, LabVantage Pharma is pre-validated in compliance with GAMP 5 guidelines.
LabVantage Pharma FAQ
How can LabVantage Pharma be executed so rapidly?
LabVantage Pharma serves as a project accelerator by reducing time and costs in two fields: validation and configuration. To pre-configure the solution, Labvantage Solutions used best-practice pharmaceutical user requirements; therefore, customers can now enjoy minimal configuration changes. The implementation, thus, concentrates on training the team on the pre-configured solution and helping users collect and load the master data necessary to work the LIMS. By strictly complying with the GAMP 5 guidelines, LabVantage Pharma easily integrates into the user’s current validation processes, reducing and simplifying the effort to validate the LIMS.
How is LabVantage Pharma a pre-validated LIMS?
LabVantage Pharma is the sole commercially available pharmaceutical LIMS solution that offers fully documented evidence of earlier validation execution. Pre-validation means it has already undergone a complete execution of the validation life cycle according to user demands, from IQ/OQ/PQ, to system release.
Other LIMS dealers do not offer completely implemented validation, but rather supply just validation test scripts. These scripts then have to be rewritten to suit the configuration and then implemented. This introduces considerable cost and probably does not cover the whole validation from the customer end. When users buy LabVantage Pharma, they obtain full evidence of the validation process for the out-of-the-box platform.
What are the components of LabVantage Pharma?
LabVantage Pharma is a comprehensive package developed for biotech and pharmaceutical quality labs. It contains everything necessary to get new, validated LIMS ready and running as soon as possible:
Software and Validation
- Fully documented proof of validation pre-executed by LabVantage
- Complete and ready-to-use validation platform
- LIMS software preconfigured especially for the pharmaceutical sector
- Reports typically used (for example, Certificate of Analysis)
- Common user roles and security descriptions
- Template document for mapping of customer products into LabVantage Pharma
- Barcode labels
Services
- System installation and arrangement — such as complete IQ testing in the customer’s space
- Master Data loading assistance for up to five product definitions, including specifications, test techniques, and stability protocols
- Time to configure for customer-defined business procedures
- Role-oriented training for strategic personnel and administrators
- Complete communication plan, including weekly status reports, project plan, risk and change management, etc.
- Comprehensive project-oriented procedure based on the Project Management Institute’s Project Management Body of Knowledge (PMBOK)
What in-built functionality is specifically meant for the pharmaceutical sector?
LabVantage Pharma leverages LabVantage 8 — a completely web-based, enterprise LIMS. It adheres to Title 21 CFR Part 11 regulatory requirements and is created, examined, and maintained using a quality system according to IEEE standards for software quality. The solution is integrated with the following functionalities:
- Stability testing and inventory organization
- Lot and batch management (full lot genealogy and quality dispositioning)
- Label printing
- End-to-end workflow with reporting and charting
- Complete sample life cycle management
- Work review routing and sign off
- Management by exception using automated calculations and specification inspection
- Dashboards and widgets
- Consumables inventory tracking including standards and reagents
- Scheduling of environmental monitoring samples
- Tracking analyst training, certification, and qualification
- Calibration, certification, and maintenance of instruments
What does pre-validation include?
LabVantage Pharma is validated to the latest version of software. Apart from the product deliverables, the package contains:
- Test strategy with completely executed verification scripts (IQ/OQ/PQ)
- Validation Master Plan created and ratified by quality and validation experts
- User requirements that encompass general industry processes and reporting
- Full set of LIMS-specific Standard Operating Procedures such as change control management and system security
- Detailed handover of the operational system including Requirements Trace Matrix, Validation Summary, Test Summary, and System Release Memo
- Documented qualifications (CVs) of specialists who created and implemented the validation
- Copies of original unimplemented test scripts for re-execution and/or alteration by the customer