2. What should I know before I use BRALTUS?
Do not use if you have ever had an allergic reaction to tiotropium, atropine, medicines
like atropine (e.g. ipratropium or oxitropium) or any of the ingredients listed at
the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines,
or are pregnant or plan to become pregnant or are breastfeeding.
3. What if I am taking other medicines?
Some medicines may interfere with BRALTUS and affect how it works.
4. How do I use BRALTUS?
The recommended dose for adults is 1 capsule to be inhaled, once a day
Inhale the powder in the capsules only using the Zonda® device. Do not swallow the capsules. Do not place a capsule directly into the mouthpiece.
Do not open the capsules.
5. What should I know while using BRALTUS?
|
Things you should do
|
Remind any doctor, dentist or pharmacist you visit that you are using BRALTUS.
Tell your doctor immediately if your breathing becomes more difficult while you are
taking BRALTUS.
|
|
Things you should not do
|
Do not use BRALTUS more frequently than once daily.
|
|
Driving or using machines
|
Be careful driving or operating machinery until you know how BRALTUS affects you.
BRALTUS may cause dizziness or blurred vision in some people.
|
|
Looking after your medicine
|
Keep your capsules in the bottle until it is time to use them.
Keep your capsules in a cool, dry place where the temperature stays below 25°C
Do not store your capsules in the refrigerator or freezer. Use the capsules within
60 days of opening the bottle
|
6. Are there any side effects?
All medicines can have side effects. If they do occur, they are usually minor and
temporary. Do not be alarmed by this list. You may not experience any of them.
A common side effect is dry mouth, which is usually mild.
Side effects that require urgent medical attention include allergic reaction, changes
in heart rate or palpitations and severe pain in the stomach with bloating, gut cramps
and vomiting.
Powder for Inhalation (in capsule)
Active ingredient(s):
Tiotropium (as bromide)
Full Consumer Medicine Information (CMI)
This leaflet provides important information about using BRALTUS. You should also speak to your doctor or pharmacist if you would like further information
or if you have any concerns or questions about using BRALTUS.
Where to find information in this leaflet:
1. Why am I using BRALTUS?
BRALTUS contains the active ingredient tiotropium (as bromide). BRALTUS belongs to a group of medicines called anticholinergics.
BRALTUS is used to make breathing easier for people with chronic obstructive pulmonary
disease (COPD).
This helps to improve your condition and to prevent exacerbations (periodic worsening
of symptoms) from occurring.
BRALTUS improves breathing by relaxing the air passages that carry air to and from
the lungs. It begins to act within 30 minutes after use and the effect should last
a full day.
2. What should I know before I use BRALTUS?
Warnings
Do not use BRALTUS to treat a sudden attack of breathlessness, wheezing or coughing. You will need a different type of medicine.
Do not use BRALTUS if:
you are allergic to tiotropium bromide, or any of the ingredients listed at the end
of this leaflet.
you are allergic to any medicine containing atropine or its derivatives, e.g. ipratropium
or oxitropium.
you are allergic to any other anticholinergic medicines.
you have been told by your doctor that you have an intolerance to some sugars, or
an allergy to milk proteins (which may be contained in small amounts in the ingredient
lactose monohydrate).
always check the ingredients to make sure you can use this medicine.
Check with your doctor if you:
Have or have had any of the following medical conditions:
High pressure in the eye (glaucoma)
Kidney or liver problems
Problems with your prostate gland
Problems with passing urine
You have suffered from a heart attack during the last 6 months or from any unstable
or life threatening irregular heart beat or severe heart failure within the past year.
During treatment, you may be at risk of developing certain side effects. It is important
you understand these risks and how to monitor for them. See additional information
under Section
6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
Your doctor can discuss with you the risks and benefits involved. BRALTUS is not generally
recommended for use in pregnant women.
Children and adolescents
Do not give this medicine to children or adolescents (below the age of 18 years).
Eyes
Do not allow the powder to enter into the eyes. Should this occur, immediately flush
your eyes with cold tap water for several minutes and immediately consult your doctor
for further advice.
If the powder enters the eye, it may result in eye pain or discomfort, blurred vision,
seeing halos around lights or coloured images in association with red eyes (i.e. narrow
angle glaucoma).
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any
medicines, vitamins or supplements that you buy without a prescription from your pharmacy,
supermarket or health food shop.
Some medicines may interfere with BRALTUS and affect how it works.
These include other anticholinergic medicines used to treat COPD such as glycopyrronium,
aclidiniuim, umeclidinium or ipratropium.
You may need different amounts of your medicines, or you may need to take different
medicines.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins
or supplements you are taking and if these affect BRALTUS.
4. How do I use BRALTUS?
How much to use
The recommended dose for adults is 1 capsule to be inhaled, once a day.
One capsule provides the required daily dose of tiotropium (a delivered dose of 10
micrograms of tiotropium); do not take more than the recommended dose.
Follow the instructions provided and use BRALTUS until your doctor tells you to stop.
When to use BRALTUS
BRALTUS should be used about the same time each day.
How to use BRALTUS
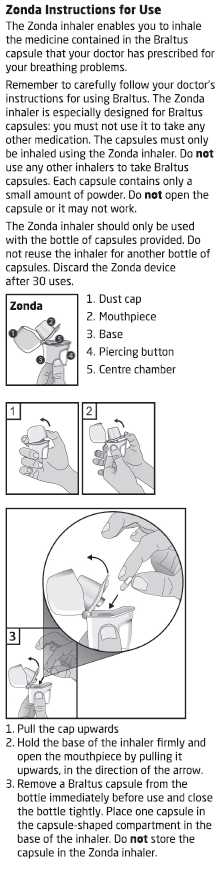
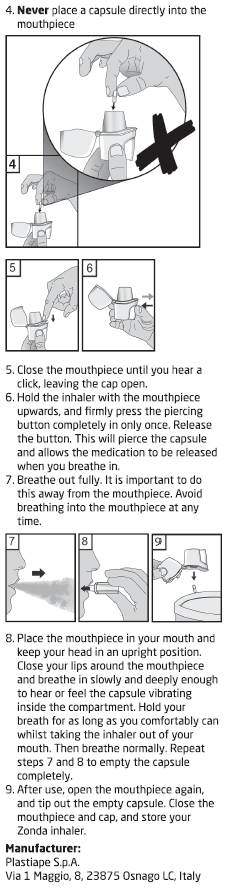
Inhale the powder in the capsules only using the Zonda device.
Do not swallow the capsules.
Do not place a capsule directly into the mouthpiece.
Do not open the capsules. If you open the capsules, the small amount of powder inside
may be lost, or you may accidentally get the powder in your eyes.
Use the capsules within 60 days of opening the bottle.
Read the Directions for Use at the end of this CMI for instructions on how to use
BRALTUS capsules with the Zonda device.
Follow the Directions for Use carefully. Pierce the capsule only once using the Zonda
device.
Do not allow the powder to enter into the eyes. Should this occur, immediately flush
your eyes with cold tap water for several minutes.
If you have any problems inhaling BRALTUS capsules using the Zonda device ask your
doctor or pharmacist for advice.
If you forget to use BRALTUS
BRALTUS should be used regularly at the same time each day.
If it is almost time for your next dose, skip the dose you missed and take your next
dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
Otherwise, take it as soon as you remember, and then go back to taking your medicine
as you would normally.
If you are not sure what to do, ask your doctor or pharmacist.
If you use too much BRALTUS
If you think that you have used too much BRALTUS, you may need urgent medical attention.
You should immediately:
phone the Poisons Information Centre
(In Australia by calling 13 11 26 or in New Zealand by calling 0800 POISON (0800 764
766)), or
contact your doctor, or
go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
Symptoms of an overdose may include fast or irregular heart beat, blurred vision,
nausea, stomach pain, dry mouth, constipation and difficulty passing urine.
5. What should I know while using BRALTUS?
Things you should do
Remind any doctor, dentist or pharmacist you visit that you are using BRALTUS, especially
if you are about to start any new medicine or if you are going to have surgery.
Keep all of your doctor’s appointments so that your progress can be checked. Your
doctor may do some tests from time to time to make sure the medicine is working and
to prevent unwanted side effects.
Follow the Directions for Use carefully.
If you are a smoker, your doctor or pharmacist can advise you on the steps to take
to quit smoking.
Call your doctor straight away if you:
become pregnant while taking this medicine
your breathing becomes more difficult while you are taking this medicine.
Things you should not do
Do not take BRALTUS to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as
you.
Do not take BRALTUS more frequently than once daily.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how BRALTUS
affects you.
BRALTUS may cause dizziness or blurred vision in some people. If you have any of these
symptoms, do not drive, operate machinery, or do anything else that could be dangerous.
Looking after your medicine
If necessary, you may wipe the mouthpiece of your Zonda inhaler after use with a dry
cloth or tissue.
The Zonda inhaler should not be rinsed with water.
Keep your capsules in the bottle until it is time to use them. If you take the capsules
out of the bottle they may not keep well.
Keep your capsules in a cool, dry place where the temperature stays below 25°C
Keep the bottle tightly closed. Store in the original package to protect from moisture.
Do not store your capsules in the refrigerator or freezer. Use the capsules within
60 days of opening the bottle.
Follow the instructions in this leaflet on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do
not store it:
in the bathroom or near a sink, or
in the car or on window sills.
Keep it where young children cannot reach it.
When to discard your medicine
Discard the Zonda device after 30 uses. Do not reuse the inhaler for another bottle
of capsules. There is a new inhaler provided with each pack of BRALTUS.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy
for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of
them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you
have any further questions about side effects.
Less serious side effects
|
Less serious side effects
|
What to do
|
|
Brain and nerves:
dizziness
trouble sleeping
Nose and sinus:
nose bleeds
sinusitis, a feeling of tension or fullness in the nose, cheeks and behind your eyes,
sometimes with a throbbing ache, fever, stuffy nose and loss of the sense of smell.
Mouth, throat and airways:
dry mouth (usually mild)
sore mouth, gums or throat
swollen, red, sore tongue
oral thrush
hoarse voice
cough
Gut and digestion:
constipation
|
Speak to your doctor if you have any of these less serious side effects and they worry
you.
|
Serious side effects
|
Serious side effects
|
What to do
|
|
Eyes:
blurred vision
seeing halos around lights or coloured images in association with red eyes;
high pressure in the eye (glaucoma)
Mouth, throat and airways:
difficulty in swallowing
a worsening of breathing problems (induced by the inhalation process). Inhaled medicines
such as BRALTUS may cause tightness of the chest, coughing, wheezing or breathlessness
immediately after inhalation.
Gut and digestion:
heartburn
Bladder:
difficulty in passing urine
pain while passing urine, urinary tract infection, increased need and frequency in
passing urine.
|
Speak to your doctor as soon as possible if you have any of these serious side effects.
|
Tell your doctor or pharmacist if you notice anything else that may be making you
feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
Always make sure you speak to your doctor or pharmacist before you decide to stop
taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What BRALTUS contains
|
Active ingredient
(main ingredient)
|
BRALTUS capsules contain 13 micrograms of tiotropium (equivalent to tiotropium bromide
15.6 micrograms) as the active ingredient.
During inhalation, 10 micrograms of tiotropium is delivered from each capsule from
the mouthpiece of the Zonda inhaler.
BRALTUS and Spiriva both deliver 10 micrograms of tiotropium and are equivalent.
|
|
Other ingredients
(inactive ingredients)
|
It also contains lactose monohydrate (which contains milk protein). The outer capsule
is an empty hard hypromellose capsule for inhalation size 3.
|
|
Potential allergens
|
lactose
|
Do not take this medicine if you are allergic to any of these ingredients.
What BRALTUS looks like
BRALTUS capsules are colourless and transparent. BRALTUS capsules contain a white
powder. The Zonda inhaler has a green body and a cap with a white push button (Aust
R 293317).
BRALTUS capsules contain only a small amount of powder, which means that the capsule
is only partially filled. The amount of powder in each capsule is equivalent in size
to the tip of a matchstick.
BRALTUS is available in a carton containing a bottle of 30 capsules with the Zonda
device.
Who distributes BRALTUS
Braltus is supplied in Australia by:
Teva Pharma Australia Pty Ltd
Level 1, 37 Epping Road
Macquarie Park NSW 2113
Braltus is supplied in New Zealand by:
Teva Pharma (New Zealand) Ltd
Auckland, New Zealand.
This leaflet was updated in May 2022