2. What should I know before I use SPRAVATO?
Do not use if you have ever had an allergic reaction to esketamine, ketamine or any
of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines,
or are pregnant or plan to become pregnant or are breastfeeding.
3. What if I am taking other medicines?
Some medicines may interfere with SPRAVATO and affect how it works.
4. How do I use SPRAVATO?
SPRAVATO nasal spray is to be used under the supervision of your doctor or other health
care professional in the doctor’s office, clinic or hospital. Your doctor will decide
how many nasal spray devices and how often you should come to the doctor’s office
or clinic for the medicine. More instructions can be found in Section
4. How do I use SPRAVATO? in the full CMI.
5. What should I know while using SPRAVATO?
|
Things you should do
|
Remind any doctor, dentist or pharmacist you visit that you are using SPRAVATO.
If you are going to have surgery, tell the surgeon or anaesthetist that you are using
SPRAVATO.
If you become pregnant or your partner becomes pregnant while using this medicine,
tell your doctor immediately.
|
|
Things you should not do
|
Do not use SPRAVATO if you are pregnant or breast-feeding.
Do not use SPRAVATO if you have, or have had an aneurysm or bleeding in the brain.
|
|
Driving or using machines
|
Do not drive, operate machinery or do anything that require you to be completely alert
until the next day following a restful sleep.
|
|
Looking after your medicine
|
Your doctor or healthcare professional will store your SPRAVATO for your use. SPRAVATO
should be kept in its original packaging in a cool dry place, where the temperature
stays below 25°C.
|
6. Are there any side effects?
The most commonly reported side effects include feeling disconnected from yourself,
your thoughts, feelings and things around you, increased blood pressure, dizziness,
headache, change in sense of taste, feeling sleepy, decreased feeling or sensitivity,
including around the mouth area, vertigo, vomiting, nausea. Call your doctor immediately
or go to the nearest hospital straight away if you have suicidal thoughts or thoughts
of harming yourself at any time, chest pain, difficulty breathing, shortness of breath,
sudden severe headache, change in vision, or seizures (fits). For more information,
including what to do if you have any side effects, see Section
6. Are there any side effects? in the full CMI.
This medicine is subject to additional monitoring. This will allow quick identification
of new safety information. You can help by reporting any side effects you may get.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems .
|
WARNING: Your healthcare professional will monitor you before and after SPRAVATO treatment
and will let you know when you can go home. They will check that your blood pressure
doesn't go too high, how sleepy you may feel, and if you are feeling disconnected
from yourself, your thoughts, feelings and/or things around you. If you are feeling
concerned about how sleepy or disconnected you feel after treatment, talk to your
healthcare professional immediately.
SPRAVATO will be provided by your healthcare professional for you to use at the clinic
or hospital under their direct supervision. SPRAVATO cannot be taken away from the
clinic or hospital. You should not drive or operate machinery until the next day after
a restful night's sleep.
|
Active ingredient(s):
esketamine hydrochloride
Full Consumer Medicine Information (CMI)
This leaflet provides important information about using SPRAVATO. You should also speak to your doctor or pharmacist if you would like further information
or if you have any concerns or questions about using SPRAVATO.
Where to find information in this leaflet:
1. Why am I using SPRAVATO?
2. What should I know before I use SPRAVATO?
3. What if I am taking other medicines?
4. How do I use SPRAVATO?
5. What should I know while using SPRAVATO?
6. Are there any side effects?
7. Product details
1. Why am I using SPRAVATO?
SPRAVATO contains the active ingredient esketamine. This belongs to a group of medicines called antidepressants and is used to treat
depression in adults.
SPRAVATO is a nasal spray used to reduce the broad range of symptoms of depression.
This medicine can help improve the symptoms of your disease and reduce the chance
of your symptoms coming back.
SPRAVATO is used in people who have tried other antidepressant medicines but have
not benefitted from them.
2. What should I know before I use SPRAVATO?
Warnings
Do not use SPRAVATO if:
you are allergic to esketamine or ketamine, or any of the ingredients listed at the
end of this leaflet.
Some of the symptoms of an allergic reaction may include shortness of breath, wheezing
or difficulty breathing, swelling of the face, lips, tongue or other parts of the
body, rash, itching or hives on the skin.
Always check the ingredients to make sure you can use this medicine.
you have, or have had, any of the following medical conditions:
an aneurysm, this is a weak spot in a blood vessel wall where it widens or bulges
out.
bleeding in the brain.
This is because SPRAVATO can cause a temporary increase in blood pressure and in both
these conditions, an increase in blood pressure can cause a serious medical condition.
Check with your doctor if you:
have or have had any of the following medical conditions:
heart problems which are not well controlled such as: poor blood flow in the blood
vessels of the heart frequently with chest pain (such as angina), high blood pressure,
a recent heart attack, heart valve disease or heart failure
slow or fast heartbeats causing shortness of breath, palpitations or chest discomfort,
feeling light-headed or fainting
problems with the blood supply to your brain (such as a stroke)
a head injury or serious problems affecting the brain, particularly where there is
increased pressure in the brain
problems with drug abuse – prescribed or illegal drugs - or a problem with alcohol
a condition called psychosis - where you believe in things that are not true (delusions)
or see, feel, or hear things that are not there (hallucinations)
you have ever had a condition called “bipolar disorder” or symptoms of mania – where
you are being very over-active or over excited
an overactive thyroid that is not properly treated (hyperthyroidism)
lung problems causing breathing difficulty (pulmonary insufficiency)
severe liver problems.
During treatment, you may be at risk of developing certain side effects. It is important
you understand these risks and how to monitor for them. See additional information
under Section
6. Are there any side effects?
Pregnancy and breastfeeding
Do not use SPRAVATO if you are pregnant.
If you are pregnant, think you may be pregnant or are planning to have a baby, tell
your doctor before using this medicine. Tell your doctor if you are trying to make
your partner pregnant.
Using SPRAVATO during pregnancy has not been studied and it is not known if SPRAVATO
will harm your unborn baby.
If you are able to become pregnant you must use highly effective contraception during
treatment - and up to 6-weeks after the end of treatment. Talk with your doctor about
methods of contraception to use.
Do not use SPRAVATO if you are breast-feeding.
The active ingredient in SPRAVATO may pass into breast milk and there is a possibility
that your baby may be affected.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any
medicines, vitamins or supplements that you buy without a prescription from your pharmacy,
supermarket or health food shop.
Some medicines may interfere with SPRAVATO and affect how it works. These include:
other nasal sprays. Do not use any other nasal spray within 1 hour of using SPRAVATO.
Central Nervous System (CNS) depressants (for example, benzodiazepines, opioids, medicines
or beverages containing alcohol)
Psychostimulants (for example, amphetamines, methylphenidate, modafanil, armodafinil)
Monoamine oxidase inhibitors (MAOIs) medicines
(for example, tranylcypromine, selegiline, phenelzine)
These medicines may be affected by SPRAVATO, or may affect how well it works. You
may need to use different amounts of your medicines, or take different medicines.
Your doctor will have more information on medicines to be careful with or avoid while
taking SPRAVATO.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins
or supplements you are taking and if these affect SPRAVATO.
4. How do I use SPRAVATO?
You will use the SPRAVATO nasal spray yourself - under the supervision of your doctor
or other health care professional in the doctor’s office, clinic or hospital.
SPRAVATO is used together with another antidepressant.
Follow all directions given to you by your doctor or other health care professional
carefully.
Your doctor or other health care professional will show you how to use the nasal spray
device and you must also read the Instructions for Use leaflet provided in the SPRAVATO
pack.
If you do not understand the instructions in the Instructions for Use leaflet, ask
your doctor or other health care professional for help.
How much to use
One nasal spray device delivers two sprays (one spray per nostril).
Your doctor will decide how many nasal spray devices and how often you should come
to the doctor’s office or clinic for the medicine.
SPRAVATO is taken twice a week for the first 4 weeks.
For some patients, after the first 4 weeks, SPRAVATO is usually taken once a week
for a further 4 weeks.
For some patients, after this, SPRAVATO is usually taken either once a week or once
every 2 weeks.
During and after each use of this medicine, you will be checked, and your doctor or
other health care professional will decide how long to monitor you.
This medicine may cause a temporary increase in your blood pressure after taking a
dose. Your blood pressure will be checked before and after taking this medicine. Tell the medical staff right away if you get chest pain, shortness of breath, sudden
severe headache, change in vision, or seizures after taking this medicine.
This medicine may cause sleepiness (sedation), fainting, dizziness, spinning sensation,
anxiety, or feeling disconnected from yourself, your thoughts, feelings, space and
time (dissociation), difficulty breathing (respiratory depression). Tell the medical staff right away if you feel like you cannot stay awake or if you
feel like you are going to pass out.
When to use SPRAVATO
You will be directed to use SPRAVATO by your doctor in the doctor’s office or clinic.
Some patients taking SPRAVATO may experience nausea or vomiting.
You should avoid eating 2 hours before and drinking liquids 30 minutes before using
this medicine.
If you need steroid or decongestant medicines as a nasal spray, avoid using these
medicines within 1 hour before your SPRAVATO treatment.
How to use SPRAVATO
Continue using SPRAVATO for as long as your doctor tells you.
Step 1.
Your doctor or other health care professional will instruct you to blow your nose
before using the first device only.
Step 2.
Your doctor or other health care professional will check the expiry date and remove
the device from its blister and check that the indicator shows 2 green dots. They
will then hand you the device.
DO NOT PRIME THE DEVICE
Step 3.
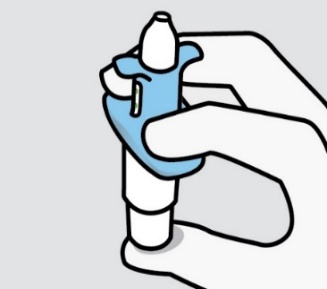
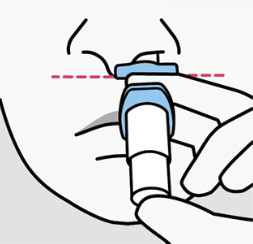
Hold device as shown with the thumb gently supporting the plunger. Do not press the
plunger.
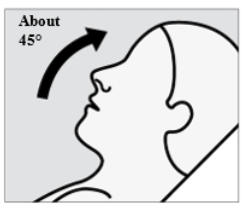
Recline head at about 45 degrees during administration to keep medication inside the
nose.
Step 4.
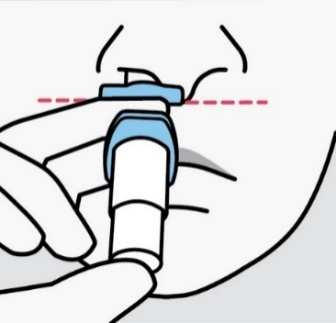
Insert tip straight into the first nostril. The nose rest should touch the skin between
the nostrils.
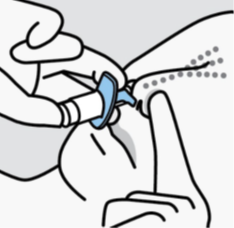
Close opposite nostril.
Breathe in through nose while pushing plunger all the way up until it stops.
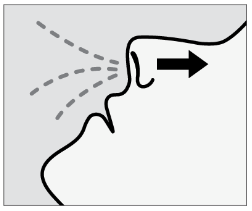
Sniff gently after spraying to keep medication inside nose.
Switch hands to insert tip into the second nostril.
Repeat Step 4 to deliver second spray.
Step 5.
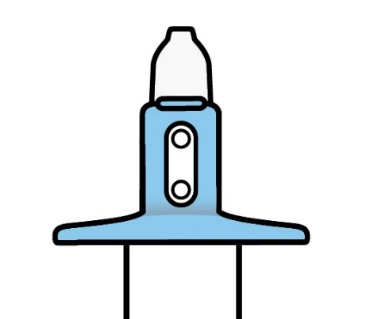
Your doctor or other health care professional will take the used device and check
that the indicator shows no green dots. If there is a green dot they will have you
spray again into the second nostril.
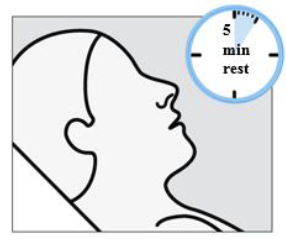
You will then rest in a semi-reclined position for 5 minutes after each device.
DO NOT BLOW NOSE.
If any liquid runs out, dab nose with a tissue.
Repeat Steps 2 - 5 if more than one device is required.
It is important that you rest for 5 minutes after each device to allow the medication
to absorb.
If you forget your appointment
It is important you make sure you come in for your scheduled appointments, so that
this medicine is effective for you.
If you miss an appointment it is important that you contact your doctor as soon as
possible to make a new appointment.
If you use too much SPRAVATO
This medicine will be given to you under the supervision of your doctor in the doctor’s
office or clinic. Therefore, it is unlikely that you will be given too much.
However, if you experience any side effects after using SPRAVATO, tell your doctor
or nurse immediately or go to Accident and Emergency at your nearest hospital.
You may need urgent medical attention.
5. What should I know while using SPRAVATO?
Things you should do
Be sure to keep all your doctor's appointments so your progress can be checked.
It is important to have your follow-up doses of SPRAVATO at the appropriate times
to get the best effects from your treatment.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking
this medicine.
If you are about to be started on any new medicine, remind your doctor and pharmacist
that you are taking SPRAVATO.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking
this medicine.
If you become pregnant or your partner becomes pregnant while taking this medicine,
tell your doctor immediately.
Tell your doctor if you are of Japanese or Chinese ancestry.
Things you should be careful of
If you think your depression getting worse
Tell your doctor or go to the nearest hospital straight away if you have suicidal
thoughts or thoughts of harming yourself at any time. You may find it helpful to talk
to a relative or close friend if you are depressed and ask them if they think your
depression is getting worse or if they are worried about your behaviour. You might
ask them to read this leaflet.
Blood Pressure
SPRAVATO can increase your blood pressure for a short time (about 1 to 2 hours) –
so you will have your blood pressure measured at various times. Your blood pressure
will be measured before you start using SPRAVATO and after taking it.
If your blood pressure is high before using this medicine, your doctor will decide
whether to start the medicine or wait until your blood pressure is lower. If your
blood pressure increases significantly after using this medicine and remains elevated
for more than a few hours after dosing, your doctor may send you to another doctor
for evaluation.
Driving or using machines
Do not drive or operate machinery after taking SPRAVATO.
SPRAVATO can make you feel sleepy, dizzy, and have other side effects that can temporarily
affect your ability to drive motor vehicles or use other machines and do anything
where you need to be completely alert. After being treated with this medicine, do
not take part in these activities until the next day following a restful sleep.
Looking after your medicine
Your doctor or healthcare professional will store your SPRAVATO for your use.
SPRAVATO should be kept in its original packaging in a cool dry place, where the temperature
stays below 25°C.
Getting rid of any unwanted medicine
Your doctor or healthcare professional will arrange to dispose of the device(s) and
any medicine that is left over.
Do not take this medicine away from the clinic.
You must never be in direct possession of this medicine outside of the treatment site.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of
them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you
have any further questions about side effects.
Less serious side effects
Serious side effects
|
Serious side effects
|
What to do
|
|
feeling disconnected from yourself, your thoughts, feelings and things around you
feeling anxious
feeling confused
difficulty in paying attention
feeling dizzy
feeling sleepy
spinning sensation (“vertigo”)
increased blood pressure
emotional distress, crying
feeling extremely happy ("euphoria")
feeling unhappy or uneasy (dysphoria)
problems with thinking
muscle tremors
difficulty speaking
fast heartbeat
excessive sweating, cold sweat
pain when passing urine
frequent need to pass urine
feeling abnormal
feeling drunk
feeling weak with low energy (asthenia)
problems walking
fast eye movements that you cannot control
making movements without purpose (psychomotor hyperactivity)
decreased feeling or sensitivity, including around the mouth area
increased saliva
low blood pressure
slow heart rate
seizure
difficulty breathing (respiratory depression)
|
Call your doctor straight away, or go straight to the Emergency Department at your
nearest hospital if you notice any of these serious side effects.
|
Tell your doctor or pharmacist if you notice anything else that may be making you
feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can
report side effects to the Therapeutic Goods Administration online at
www.tga.gov.au/reporting-problems . By reporting side effects, you can help provide more information on the safety of
this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop
taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What SPRAVATO contains
|
Active ingredient (main ingredient)
|
esketamine hydrochloride
Each nasal spray device contains 32.3 mg of esketamine hydrochloride (equivalent to
28 mg esketamine)
|
|
Other ingredients
(inactive ingredients)
|
citric acid monohydrate
disodium edetate
sodium hydroxide
water for injections
|
Do not take this medicine if you are allergic to any of these ingredients.
SPRAVATO does not contain any preservatives, lactose, sucrose, gluten, tartrazine
or any other azo dyes.
What SPRAVATO looks like
SPRAVATO is a nasal spray solution (AUST R 311827).
It is a clear, colourless, aqueous solution provided in a prefilled nasal spray device.
Each nasal spray device is individually packaged in a sealed blister pack.
SPRAVATO is available in pack sizes containing 1, 2, or 3 single use nasal spray devices.
Who distributes SPRAVATO
JANSSEN-CILAG Pty Ltd
17 Khartoum Road,
Macquarie Park,
NSW 2113, AUSTRALIA
Telephone: 1800 226 334
NZ Office: Auckland New Zealand
Telephone: 0800 800 806
This leaflet was prepared in December 2025.