Introduction
Virology and pathogenesis
Ecological drivers of emergence
Surveillance and detection
Prevention and control strategies
Veterinary interventions
Human risk mitigation
Ecological interventions
Broader zoonotic implications
Research gaps and future directions
Conclusion
References
Further reading
Climate change and habitat disruption are bringing bats, horses, and humans closer, fueling the spread of the Hendra virus. Discover how a One Health approach can break the chain of transmission and protect against future outbreaks.
 Hendra virus (HeV). Image Credit: Corona Borealis Studio / Shutterstock.com
Hendra virus (HeV). Image Credit: Corona Borealis Studio / Shutterstock.com
Introduction
Hendra virus (HeV) is a member of the Henipavirus genus in the Paramyxoviridae family. HeV was initially discovered in 1994 during an outbreak of fatal respiratory illness in horses in Hendra, a suburb of Brisbane, Australia. The outbreak involved 21 horses, 14 of which died or were euthanized, as well as two human infections, including one fatality.
Since the discovery of HeV, sporadic outbreaks have been reported throughout Queensland and New South Wales, primarily involving transmission from flying foxes to horses and occasionally from horses to humans. One case in a dog has also been documented, thus highlighting the potential for cross-species transmission of HeV.1,2
Virology and pathogenesis
HeV is an enveloped, pleomorphic virus, with morphology ranging from spherical particles to elongated filaments. Both the attachment glycoprotein (G) and fusion protein (F) protrude outward from the viral membrane, enabling host cell entry through ephrin B2 and B3 receptors.
The single-stranded, negative-sense, unsegmented viral ribonucleic acid (RNA) genome is bound by nucleocapsid proteins (N), which form a ribonucleoprotein (RNP) complex that antagonizes innate immune responses.4,5
The natural reservoir of HeV is the Pteropus species of fruit bats, which are otherwise known as flying foxes, in addition to P. alecto, P. poliocephalus, P. scapulatus, and P. conspicillatus species that also harbor HeV.6 Viral spillover to horses typically occurs when animals ingest or inhale food or water contaminated with bat excreta or urine.
Human infection occurs through close contact with infected horses and their bodily fluids, such as blood, nasal discharge, and saliva. To date, no human-to-human transmission has occurred.
Although rare, human disease is often severe and presents with influenza-like symptoms that may progress to encephalitis or respiratory failure. With a case fatality rate of 57% and no approved treatments, HeV remains a high-priority zoonotic pathogen requiring biosafety level 4 (BSL4) measures.7
Ecological drivers of emergence
HeV emergence is associated with ecological disruption, particularly habitat loss and climate change. Extensive land clearing for agriculture, mining, and urban development has fragmented natural habitats, thereby displacing flying foxes and increasing their proximity to human and horse populations.8
Drought, food scarcity, and stress from extreme temperatures disrupt patterns of bat foraging, migration, and roosting. This displacement forces flying foxes into orchards, paddocks, and backyards where horses can ingest contaminated excreta.3,8
Climate change is worsening high-risk spillover zones for HeV by shifting bat ranges, altering flowering patterns, and intensifying droughts. Poor eucalyptus flowering and habitat loss force flying foxes into urban areas, further increasing stress and viral shedding.
Each of these factors increases the risk of spillover events, particularly during foaling season between August and December, which overlaps with the winter season in eastern Australia. The southward movement of Pteropus alecto into horse-dense regions, such as New South Wales’ Upper Hunter, highlights how climate-driven ecological changes are escalating zoonotic threats.9
Surveillance and detection
Effective surveillance and early detection of HeV can prevent spillover events, thereby protecting human and animal health. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) assay can be used to measure HeV antigen levels in blood samples or swabs obtained from horses within four hours.
Virus isolation from nasopharyngeal or oropharyngeal swabs, as well as clotted blood samples, can also confirm the presence of HeV. Enzyme-linked immunosorbent assays (ELISAs) can also be used to detect anti-HeV antibodies in clotted blood, providing evidence of past exposure or infection to aid in epidemiological tracking.10 Moreover, serological surveillance in bats and horses allows researchers to identify high-risk areas to subsequently inform vaccination and biosecurity strategies.
Subclinical and intermittent infections in bats, as well as nonspecific or sudden symptoms in horses, complicate diagnosis and prevent the early detection of HeV. Limited bat surveillance, especially of key species like Pteropus Alecto, as well as inadequate diagnostic access in rural areas, can also lead to significant underreporting.10,11
 Image Credit: CHANUN.V / Shutterstock.com
Image Credit: CHANUN.V / Shutterstock.com
Prevention and control strategies
Veterinary interventions
The Equivac® HeV subunit vaccine, which consists of a soluble G glycoprotein, was initially developed in 2012 to prevent HeV transmission in horses. This vaccine schedule consists of two initial doses, a third dose administered six months later, and annual boosters. By inducing the production of protective antibodies, the Equivac® vaccine protects horses from fatal infection while simultaneously interfering with the bat-horse-human transmission chain.11
However, vaccine uptake remains low due to mistrust of pharmaceutical companies, cost concerns, perceived low risk, and limited awareness. Misunderstandings about safety and efficacy further deter use. Addressing these barriers through transparent communication, incentives, and veterinary-led outreach could improve coverage while also protecting animal and human health in high-risk regions.12
Human risk mitigation
Human risk mitigation for HeV is dependent upon the strict use of personal protective equipment (PPE) during equine care and autopsy, as infected horses can shed the virus, even before symptoms emerge. Full PPE that includes gloves, respirators or masks, gowns, face shields, and protective footwear is essential to protect veterinarians and handlers from exposure.13
Ongoing public health education for veterinarians and horse owners is also crucial. Key efforts include recognizing early symptoms, promoting vaccination, implementing basic biosecurity, and ensuring proper PPE use through regular training. Open communication fosters trust and encourages timely reporting, which reduces the risk of spillover.14
Australian scientists closer to Hendra virus vaccine thanks to an alpaca named Pedro | 7.30
Ecological interventions
Preventing HeV spillover requires ecological interventions that reduce contact between flying foxes and domestic animals. Preserving and restoring native bat habitats, such as replanting fruiting and flowering trees away from farms, can redirect bats from foraging near horses, thereby reducing the risk of viral shedding in paddocks.14
Establishing buffer zones between bat roosts and horse areas through fencing, relocating horses during peak bat activity, and placing feed and water away from bat-frequented trees can also be effective. These measures, combined with farm-level biosecurity practices, are crucial in high-risk regions to minimize the risk of spillover.12,14
Broader zoonotic implications
HeV and Nipah virus (NiV) are closely related members of the Henipavirus genus that cause severe zoonotic outbreaks. Whereas HeV primarily affects horses and humans in Australia, NiV has led to fatal human infections in Southeast Asia, with mortality rates reaching 100% in some outbreaks.5,15
Genetic similarities between HeV and NiV, coupled with their broad host range, raise concerns about viral evolution or recombination. This is particularly worrisome amid habitat loss, climate change, and increased cross-species contact that create ideal conditions for adaptation and potential human-to-human transmission.
These risks underscore the importance of a One Health approach, which considers the interconnectedness of animal, human, and environmental health through coordinated surveillance, ecological protection, vaccination, and education, to prevent future spillovers.15
Research gaps and future directions
Spillover prediction models must incorporate climate data, bat ecology, temperature, landscape forest cover, land-use changes, human population density, and horse management practices. Although a licensed equine vaccine is available, there is no approved human vaccine or antiviral therapy. Promising candidates, such as a subunit human vaccine and the monoclonal antibody m102.4, are still being evaluated for their safety, efficacy, and long-term protection as post-exposure treatments.15,16
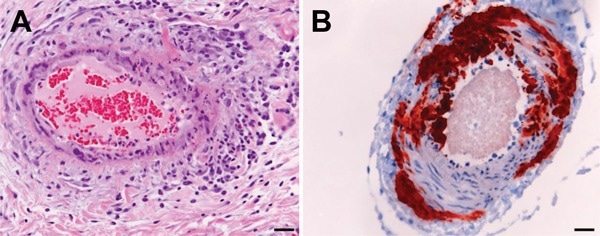
Histologic and immunohistologic findings in Hendra virus–infected horse tissue. A) Hematoxylin and eosin staining shows systemic vasculitis affecting the lung. B) Immunohistologic examination, using polyclonal rabbit anti-Nipah N protein, indicates Hendra virus antigen in a blood vessel in the brain. Scale bars represent 50 μm. 15
Longitudinal research is also needed to elucidate how climate change, nutritional stress, bat reproductive cycles, and habitat fragmentation influence viral shedding and transmission. These insights can be used to refine outbreak prediction and prevention strategies.
International collaboration is essential to mitigate HeV outbreaks, given the global distribution of bats and their role in the emergence of viral zoonoses. Strengthening cross-border surveillance, data sharing, and coordinated research on diagnostics, vaccines, and treatments will enhance global readiness against HeV and other bat-borne pathogens.16
Conclusions
Preventing future HeV outbreaks requires proactive, transdisciplinary action. Surveillance efforts must include ecological monitoring of reservoir species to enable early warning. Protecting and restoring bat habitats can further reduce human–animal interface and address root causes of spillover.
A One Health approach, which combines veterinary science, ecology, climatology, public health, and social sciences, is essential for developing and implementing effective prevention strategies. Integrating ecological preservation with targeted surveillance and cross-sector collaboration has the potential to build stronger resilience against HeV and other emerging zoonotic threats driven by global environmental change.
References
- Christopher C. Broder et al. (2013). A treatment for and vaccine against the deadly Hendra and Nipah viruses. Antiviral Research, 100 (1):8-13. DOI: 10.1016/j.antiviral.2013.06.012, https://www.sciencedirect.com/science/article/pii/S0166354213001691
- Yuen KY et al. (2021). Hendra virus: Epidemiology dynamics in relation to climate change, diagnostic tests and control measures. One Health,12:100207. DOI: 10.1016/j.onehlt.2020.100207. https://www.sciencedirect.com/science/article/pii/S2352771420303086?via%3Dihub
- Kessler MK et al. (2018). Changing resource landscapes and spillover of henipaviruses. Ann N Y Acad Sci., 1429(1):78-99. DOI: 10.1111/nyas.13910. https://nyaspubs.onlinelibrary.wiley.com/doi/10.1111/nyas.13910
- Christina L. Faust. (2023). Environmental variation across multiple spatial scales and temporal lags influences Hendra virus spillover. Journal of Applied Ecology, 60, 1457–1467. DOI: 10.1111/1365-2664.14415. https://besjournals.onlinelibrary.wiley.com/doi/10.1111/1365-2664.14415
- Broder CC, Weir DL, Reid PA. (2016). Hendra virus and Nipah virus animal vaccines. Vaccine, 34 (30) 3525-34. DOI: 10.1016/j.vaccine.2016.03.075. https://www.sciencedirect.com/science/article/abs/pii/S0264410X1630072X
- Walsh, M.G., Wiethoelter, A. & Haseeb, M.A. (2017). The impact of human population pressure on flying fox niches and the potential consequences for Hendra virus spillover. Sci Rep 7, 8226. DOI: 10.1038/s41598-017-08065-z. https://www.nature.com/articles/s41598-017-08065-z
- Baranowski K, Bharti N. (2023). Habitat loss for black flying foxes and implications for Hendra virus. Landsc Ecol., 38(6):1605-1618. DOI: 10.1007/s10980-023-01642-w. https://link.springer.com/article/10.1007/s10980-023-01642-w
- Eby P et al. (2023). Pathogen spillover driven by rapid changes in bat ecology. Nature, 613(7943):340-344. DOI: 10.1038/s41586-022-05506-2. https://www.nature.com/articles/s41586-022-05506-2
- Goldspink LK, Edson DW, Vidgen ME, Bingham J, Field HE, and Smith CS. (2015). Natural Hendra Virus Infection in Flying-Foxes - Tissue Tropism and Risk Factors. PLoS One, 10(6):e0128835. DOI: 10.1371/journal.pone.0128835. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0128835
- Edson D et al. (2015). Routes of Hendra Virus Excretion in Naturally-Infected Flying-Foxes: Implications for Viral Transmission and Spillover Risk. PLoS ONE, 10(10): e0140670. DOI: 10.1371/journal.pone.0140670. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0140670
- Hazelton, B., Ba Alawi, F., Kok, J., and Dwyer, D. E. (2013). Hendra Virus: A One Health Tale of Flying Foxes, Horses and Humans. Future Microbiology, 8(4), 461–474. DOI: 10.2217/fmb.13.19. https://www.tandfonline.com/doi/10.2217/fmb.13.19
- Jessica N Kropich-Grant, Kerrie E Wiley, Jennifer Manyweathers, Kirrilly R Thompson, and Victoria J Brookes. (2023). Communication Interventions and Assessment of Drivers for Hendra Virus Vaccination Uptake. Vaccines, 11, 936. DOI: 10.3390/ vaccines11050936. https://www.mdpi.com/2076-393X/11/5/936
- Middleton D. (2014). Hendra virus. Vet Clin North Am Equine Pract., 30(3):579-89. DOI: 10.1016/j.cveq.2014.08.004. https://www.sciencedirect.com/science/article/abs/pii/S0749073914000625?via%3Dihub
- Plowright RK et al. (2024). Ecological countermeasures to prevent pathogen spillover and subsequent pandemics. Nat Commun., ;15(1):2577. DOI: 10.1038/s41467-024-46151-9. https://www.nature.com/articles/s41467-024-46151-9
- Middleton D et al. (2014). Hendra virus vaccine, a one health approach to protecting horse, human, and environmental health. Emerg Infect Dis.;20(3):372-9. DOI: 10.3201/eid2003.131159. https://wwwnc.cdc.gov/eid/article/20/3/13-1159_article
- Jagadesh S. et al. (2025). Mapping global risk of bat and rodent-borne disease outbreaks to anticipate emerging threats. Sci Rep.;15(1):20534. DOI: 10.1038/s41598-025-05588-8. https://www.nature.com/articles/s41598-025-05588-8
Further Reading
Last Updated: Jul 22, 2025