This article is based on a poster originally authored by Fiona Leslie, Chloe Whiting, and Megan Webster.
Idiopathic Pulmonary Fibrosis (IPF) is a chronic and progressive interstitial lung disease characterized by damage to lung architecture, resulting in an irreversible loss of function.
The etiology of IPF remains unknown; however, it is believed to stem from epithelial damage, abnormal epithelial-fibroblast communication, and a subsequent dysregulated tissue repair response.
TGF-β1-mediated activation of fibroblasts, their transition to α-SMA-expressing myofibroblasts, and the associated increase in the expression and deposition of extracellular matrix proteins represent key pathological mechanisms underlying fibrosis.
Modeling this process in vitro facilitates the evaluation of potential anti-fibrotic therapeutics for their efficacy in reducing or reversing the fibroblast-to-myofibroblast transition (FMT).
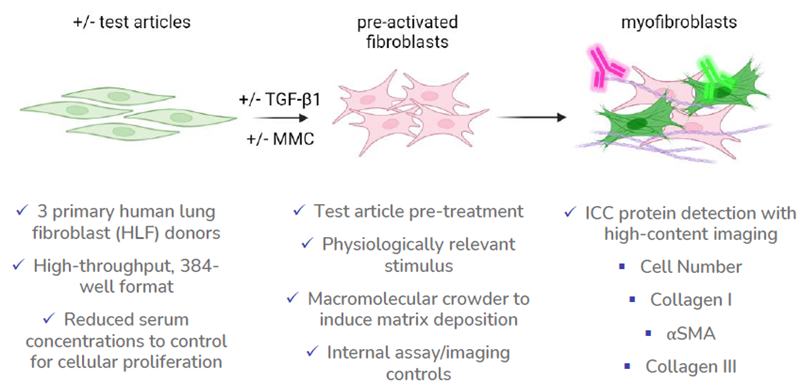
Image Credit: Newcells Biotech
Assay characterization
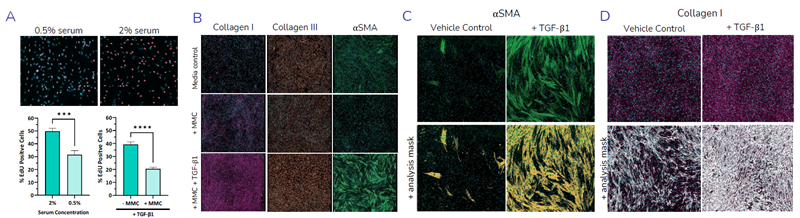
Assay characterization showing the effects of serum concentration and macromolecular crowding agents on cell proliferation and the expression of fibrosis related proteins collagen I, collagen III, and αSMA expression in primary HLF cells with examples of imaging analysis by application of segmentation masks. Image Credit: Newcells Biotech
Lung fibroblasts upregulate fibrotic markers in response to TGF-β1
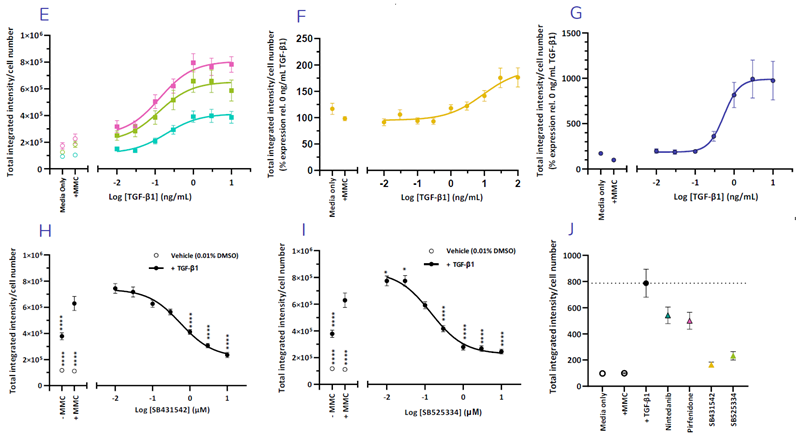
Effects of increasing TGF-β1 concentration on the expression of collagen I (E), collagen III (F) and αSMA (G) expression in normal HLFs. TGF-β1 induced collagen I expression is dose-dependently reduced by ALK5 inhibitors SB431512 and SB525334 (H & I). Physiologically relevant concentrations of Nintedanib (100 nM) and Pirfenidone (100 μM) reduce collagen I expression (J). Image Credit: Newcells Biotech
Newcells Biotech’s FMT assay
Newcells’ FMT assay employs high-content imaging to assess the effects of potential therapeutics on fibroblast activation as well as collagen expression and deposition in a high-throughput format.
Assay features:
- Three validated primary HLF donors
- High-throughput 384-well format
- Six-point dose response for up to six TAs per plate
- Six technical replicates per condition
- Validated experimental assay controls
Available assay readouts:
- Cell Number
- Collagen I
- αSMA
- Collagen III
Acknowledgments
Produced from material originally authored by Fiona Leslie, Chloe Whiting and Megan Webster from Newcells Biotech.
About Newcells Biotech
Newcells Biotech develops in vitro cell-based assays for drug and chemical discovery and development.
Using our expertise in induced pluripotent stem cells (iPSCs), cellular physiology, and organoid technology, we build models that incorporate the “best biology” for predicting in vivo behavior of new drugs.
Our experts have developed and launched assays to measure transporter function, safety, and efficacy in a range of cell and tissue types, including kidney, retina and lungs.
We have the capability to develop and implement protocols to measure cilia beat frequency and toxicity on small airway epithelial cells model, retinal toxicity and disease modelling on retinal organoids and retina epithelium, as well as drug transport in the kidney, DDI and nephrotoxicity across human and a range of preclinical species.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: May 13, 2025