This article is based on a poster originally authored by Colin D.A Brown, Fiona Leslie, Oliver Birch, Chloe Whiting, and Megan Webster.
Inhaling toxic microparticles can damage the lower airway epithelial cells. Prolonged microinjury to the epithelium results in abnormal communication between epithelial cells and fibroblasts, mediated by the release of fibrotic stimuli such as TGFβ1.
TGF-β1 induces the phenotypic transformation of fibroblasts into myofibroblasts (FMT). Activated myofibroblasts are responsible for the excessive accumulation of extracellular matrix (ECM) proteins, which is a hallmark of fibrosis.
This process results in irreversible damage to lung architecture and function. Consequently, there is a pressing need for novel anti-fibrotic therapies to mitigate disease progression.
Modeling FMT processes in vitro through high-content imaging facilitates compound screening to support anti-fibrotic drug discovery.
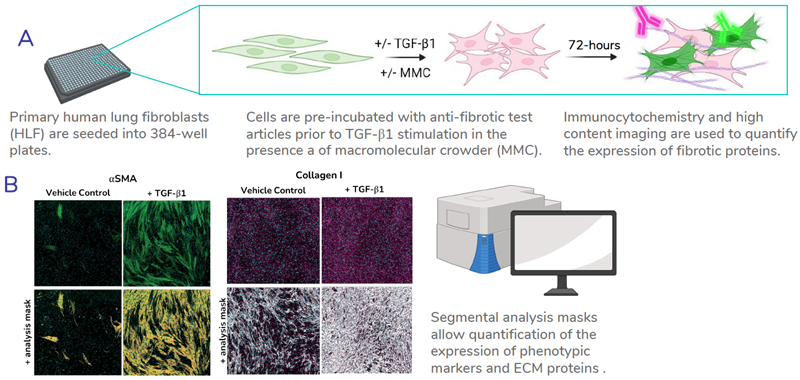
Figure 1. High throughput assay setup. A) Schematic outlining the process of the FMT assays. B) Representative images showing the segmental analysis masks used for quantification of fibrotic proteins. Examples shown: α-smooth muscle actin (α-SMA) and collagen I. Image Credit: Newcells Biotech
Optimization of culture conditions for assay robustness
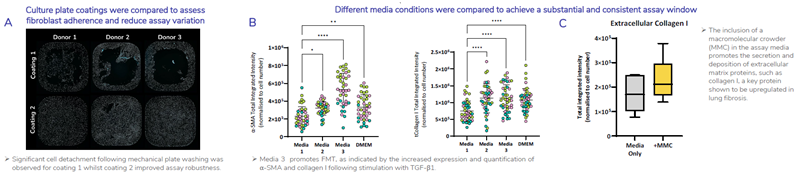
Figure 2. FMT assay conditions have been optimized to ensure reproducibility and achieve the greatest assay window to allow the study of potential anti-fibrotic therapeutics. A) 384-well plates were coated with two different coatings and HLF were seeded into wells at an equal density. Cells were fixed and stained using Hoechst 33342 to detect cell nuclei representative images shown of whole well x4 magnification. B) Four assay medias were compared to determine which assay media promotes FMT to provide a robust and significant assay window. HLF were stimulated with TGF-β1 in four different assay medias. Shown is total integrated intensity normalized to cell number for a-SMA (left) and collagen I (right) of HLF (N=3 donors, each colour point represents a donor) stimulated with 1ng/mL TGF-β1. Statistical analysis performed; one-way ANOVA with Dunnett’s multiple comparisons test compared to Media 1. C) Inclusion of a macromolecular crowding agent (MMC) in the culture media promotes deposition of extracellular matrix and shows an increase in the baseline expression of extracellular collagen I. Image Credit: Newcells Biotech
TGF-β1 induces FMT in normal and IPF donors
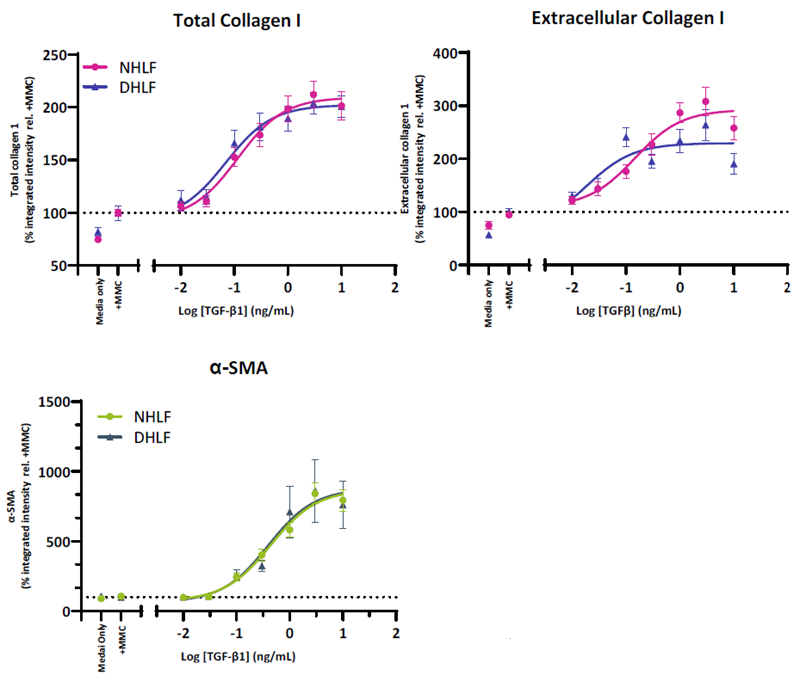
Figure 3. HLF from both normal and IPF donors demonstrate a TGF-β1 driven increase in FMT markers. Collagen I is increased in both the intracellular and extracellular compartments whilst α-SMA incorporation into stress fibres is indicative of fibroblast activation. HLF from normal (NHLF) and diseased (DHLF) donors were seeded into 384-well plates. The following day cells were stimulated with TGF-β1 dose response prepared in assay media and cultured for 72-hours. Cells were fixed and immunostained for collagen I (total and extracellular) and α-SMA. Shown is the total integrated intensity normalized to cell number expressed relative to the media control combined for N=3 NHLF and N=2 DHLF donors. IPF-Idiopathic Pulmonary Fibrosis. Image Credit: Newcells Biotech
Inhibition of FMT using ALKi: SB525433
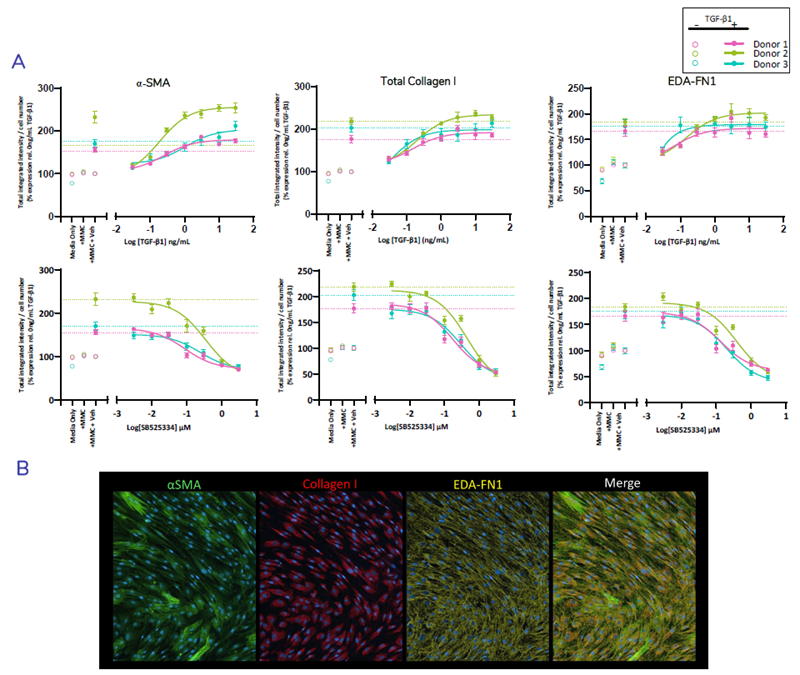
Figure 4. Multiplexed detection and quantification of fibrosis proteins αSMA, collagen I, and the EDA-splice variant of Fibronectin (EDA-FN1)* Normal HLF (N=3 donors) were seeded into 384-well plates. Cells were pre-treated for 1 hour ± SB525334 over 7-point dose-response prepared in assay media prior to stimulation with TGF-β1. Cells were cultured for 72-hours, fixed and stained using multiplexed antibodies directed against α-SMA, total collagen I and EDA-fibronectin was performed. Quantification showing % change in total integrated intensity relative to the vehicle control for each marker normalised to cell number (A) and representative images of stained FMT markers in TGF-β1 stimulated cells (B). TGF-β1 dose response (top) and inhibition with ALK5i SB525334 (below). Dashed line represent TGF-β1 spiked vehicle control. Error bars represent ±SEM. n=2 experimental repeats, n=12 technical replicates. Image Credit: Newcells Biotech
Newcells Biotech’s FMT assay for evaluating anti-fibrotic therapeutics
Newcells Biotech’s FMT assay employs high-content imaging to assess the impact of therapeutics on disease-relevant markers of fibroblast activation (α-SMA) and ECM protein expression (collagen I and EDA-FN1).
Acknowledgments
Produced from material originally authored by Colin D.A Brown, Fiona Leslie, Oliver Birch, Chloe Whiting and Megan Webster from Newcells Biotech.
About Newcells Biotech
Newcells Biotech develops in vitro cell-based assays for drug and chemical discovery and development.
Using our expertise in induced pluripotent stem cells (iPSCs), cellular physiology, and organoid technology, we build models that incorporate the “best biology” for predicting in vivo behavior of new drugs.
Our experts have developed and launched assays to measure transporter function, safety, and efficacy in a range of cell and tissue types, including kidney, retina and lungs.
We have the capability to develop and implement protocols to measure cilia beat frequency and toxicity on small airway epithelial cells model, retinal toxicity and disease modelling on retinal organoids and retina epithelium, as well as drug transport in the kidney, DDI and nephrotoxicity across human and a range of preclinical species.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: May 13, 2025