This article is based on a poster originally authored by Kun Shi, Na Zhang, Xing Zhang, Spencer Chiang, Haonan Li, and Tianfu Zhang.
Natural killer (NK) cells constitute the third largest lymphocyte subset, representing around 10-15% of circulating lymphocytes in blood. These large granular cells are essential to the immune system’s anti-inflammatory response and tumor surveillance.
The need to obtain a sufficient number and quality of NK cells represents a potentially significant bottleneck in the application of NK cells in adoptive immunotherapy.
The advent of induced pluripotent stem cells (iPSCs) could be key to addressing this challenge, with iPSCs offering the potential for self-renewal and pluridirectional differentiation. iPSCs can also be induced to differentiate into NK cells and a range of cell types in vitro.
ACROBiosystems has successfully developed a robust and novel method for inducing NK cells from iPSCs under entirely serum-free conditions. Using this approach, the proportion of CD3-CD56+ NK cells reached 85.9% after 25 days of induction.
Developed NK cells demonstrate a similar biological function to NK cells generated via other sources, meaning that these cells represent a feasible means of scaling up the manufacturing of NK cells for a diverse array of clinical applications.
iPSC to NK cell workflow
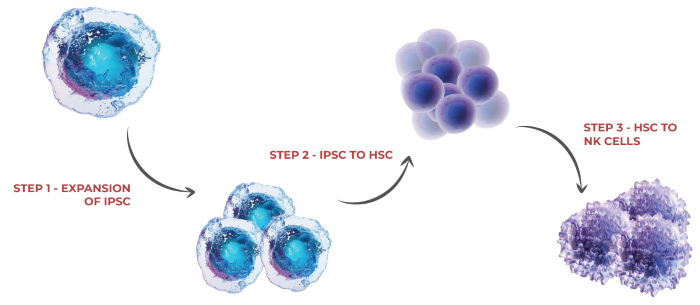
Image Credit: ACROBiosystems
Step 1: Expansion of iPSCs
iPSCs can function as a foundational resource due to their capacity for indefinite self-renewal in culture while maintaining their pluripotency. This characteristic allows researchers to generate considerable quantities of high-quality, uniform cells required for therapeutic applications.
Several aspects must be monitored during this step to ensure high-quality iPSC culture. These include stemness, expansion fold, and genetic uniformity. It is also important to use well-defined raw materials such as recombinant laminin 521. These materials negate the need to use animal-origin materials, and their GMP grade helps to ensure efficacy and safety.
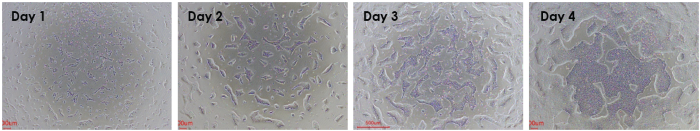
Figure 1. Laminin 521 used as a coating substrate for the fast expansion of single cell human PSCs across 4 days. Image Credit: ACROBiosystems
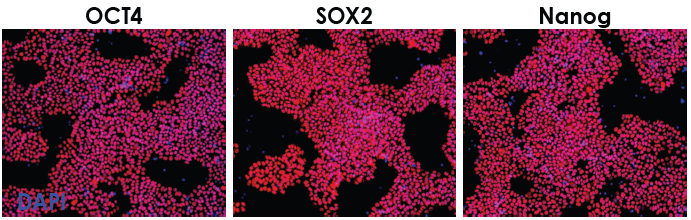
Figure 2. Stemness markers OCT4, SOX2, and Nanog are present after several passages, showing robust self- renewal of hPSCs. Image Credit: ACROBiosystems
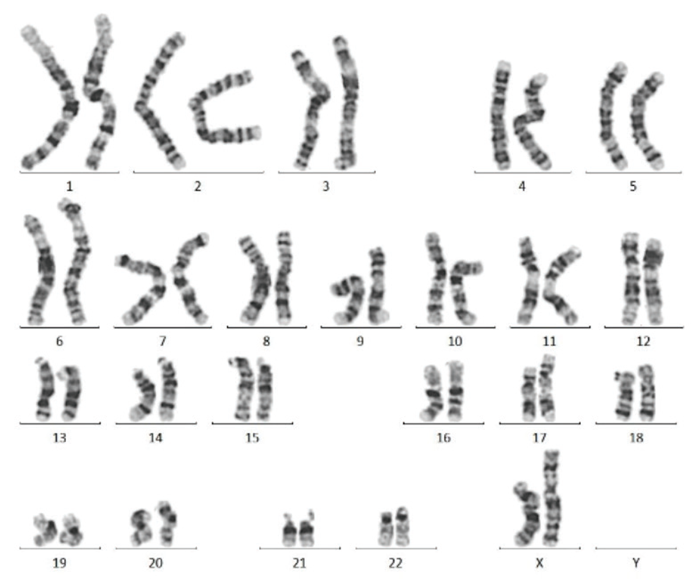
Figure 3. Karyotype analysis after 10 passages reveals a normal karyotype in iPSCs. Image Credit: ACROBiosystems
Source: ACROBiosystems
| |
|
Solutions:
- Laminin 521
- Laminin 511
- Vitronectin
- FGF basic
- TGF-β1
- Mogengel BME
|
Step 2: iPSC to HSC differentiation
Human-induced pluripotent stem cells were initially digested into single cells to form embryoid bodies. Embryoid bodies were then cultured in a series of mediums containing VEGF, bFGF, BMP4, SCF, TPO, Flt-3L, and other factors for 14 days. Finally, flow cytometry was used to determine the expression of CD34 and CD45 to ensure the HSC population.
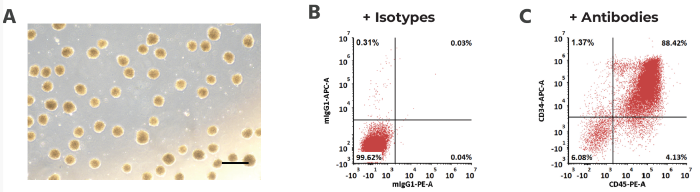
Figure 4. (A) Embroyoid bodies formed by human induced pluripotent stem cells. Scale bar, 250 μm. (B,C) CD34+ CD45+ hematopoietic cell acquisition by FACS. Image Credit: ACROBiosystems
Source: ACROBiosystems
| |
|
Solutions:
- BMP4
- VEGF
- FGF-basic
- SCF
- TPO
- FLT-3L
|
Step 3: HSC to NK cell differentiation
Human CD34+ hematopoietic cells were seeded in pre-coated wells and cultured for 21 days in a medium containing TPO, Flt-3L, SCF, IL-7, and other factors.
CD5 and CD7 expression were analyzed via flow cytometry. Select Lymphoid Progenitor Cells (LPCs) were seeded into precoated wells before further culturing in a medium containing FLT3L, TPO, SCF, IL-2, IL-3, IL-7, and IL-15, as well as either or both DLL4 and VCAM1.
It is important to note that adding small molecules can also be effective in NK expansion, considerably improving cell viability.
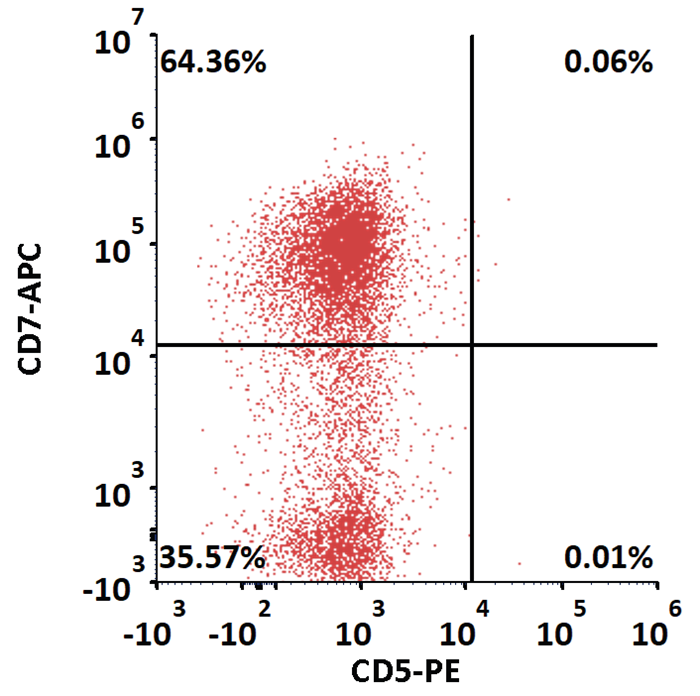
Figure 5. CD7+ CD5- Lymphoid Progenitor Cell (LPC) differentiated from HSC evaluation on Day 21. DLL4 and VCAM1 were used as a supportive environment to drive differentiation to NK cell lineage. Image Credit: ACROBiosystems
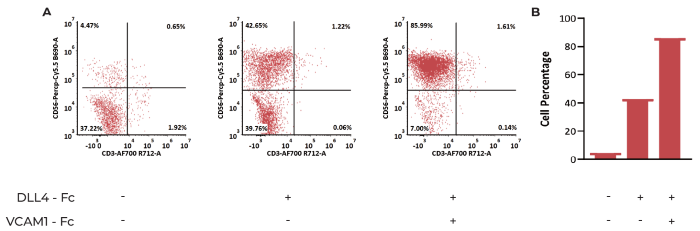
Figure 6. Hematopoietic cells differentiate to CD3- CD56+ NK Cells after 25 days of Culture (A) Flow cytometry assessment of the percentage of NK cells. (B) Statistics of the percentage of NK cells under three different conditions. The results indicate that VCAM1 and DLL4 promotes NK cell differentiation. Image Credit: ACROBiosystems
Source: ACROBiosystems
| |
|
Solutions:
- DLL4
- VCAM1
- SCF
- FLT3L
- TPO
- IL-2
- IL-3
- IL-7
- IL-15
|
Cytotoxicity, degranulation, and secreted cytokine analysis of developed NK cells
NK cells were co-cultured with cells derived from K562. This was done at E:T ratios of 0.625:1, 1.25:1, 2.5:1, 5:1, and 10:1.
Next, 7-AAD/CFSE staining was used to measure cytolysis of target cells, and these were tested via flow cytometry. NK cells’ cytotoxicity was found to increase in line with the increment of the E:T ratio, with more than 40% of target cells lysed even at a low ratio of E:T=0.625:1.
The level of CD107a on the NK cells was analyzed prior to and following coculturing with K562 cells to evaluate the developed NK cells further. CD107a expression was determined to increase following coculturing with K562, indicating that NK cells were secreting more granzyme and perforin.
NK and K562 cells were also cocultured for a total of 4 hours at an E:T ratio = 10:1. The supernatant was measured for IFN-γ using the ClinMax™ Human IFN-γ ELISA kit. These measurements revealed that NK cells co-cultured with K562 secreted greater levels of IFN-γ than the blank group.
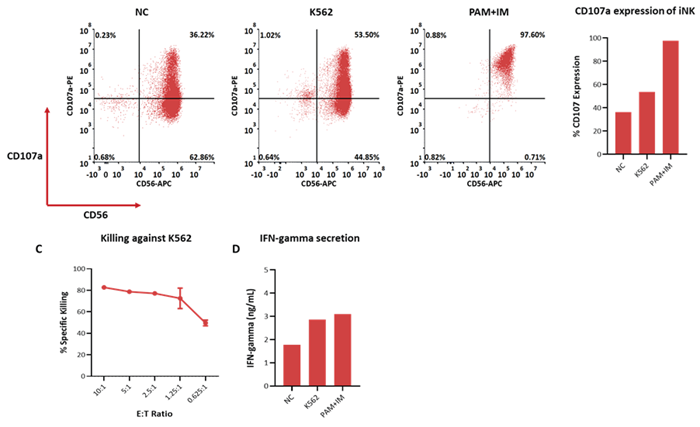
Figure 7. Cytotoxicity, Degranulation marker expression and cytokine production of iNK cells after exposure to K562 cells. (A, B) degranulation marker CD107a in iNK cells after coculture with K562 cells detected by flow cytometry. (C) Cytolysis of K562 cells was done with 7-AAD/CFSE staining and tested by flow cytometry. Spontaneous death of target cells has been subtracted from all plots. (D) IFN -γ secreted by iNK cells after exposure to K562 were quantified through ELISA assay. Image Credit: ACROBiosystems
Conclusion
CAR-NK offers significant potential in cell therapy, as evidenced by its distinctive safety and manufacturing benefits. However, finding a scalable, sustainable, and uniform source of NK cells is essential to take advantage of NK cell therapies' higher patient response.
iPSCs are key to this process, enabling reproducible expansion and differentiation into NK cells while continuing to be able to trace their source. In the example presented here, it was necessary to perform extensive validation of the final cell phenotype at each step. The highlighted protocol for iPSC to NK differentiation is scalable, meaning that it is ideally suited to cell therapy manufacturing.
References and further reading
- Fares, I., & Yzaguirre, P. (2018). Small molecules for human hematopoietic stem cell expansion and differentiation. Current Opinion in Hematology, 25(4), 318-324.
- Bhatia, M., & Wang, J. C. (2016). Clinical translation of haematopoietic stem cell expansion: achievements and challenges. Nature Reviews Drug Discovery, 15(10), 709-723.
- Small molecule regulators for the ex vivo expansion of hematopoietic stem and progenitor cells. Journal of Hematology & Oncology, 10(1), 1-11.
- Boitano, A.E., et al. (2010). Aryl Hydrocarbon Receptor Antagonists Promote the Expansion of Human Hematopoietic Stem Cells. Science, (online) 329(5997), pp.1345–1348. https://doi.org/10.1126/science.1191536.
- Cutler, C., & Antman, K. H. (2004). The use of hematopoietic growth factors and cytokines in cancer treatment. CA: A Cancer Journal for Clinicians, 54(3), 150-161.
- Maskalenko, N.A., Zhigarev, D. and Campbell, K.S. (2022). Harnessing natural killer cells for cancer immunotherapy: dispatching the first responders. Nature Reviews Drug Discovery, 21(8), pp.559–577. https://doi.org/10.1038/s41573-022-00413-7.
- Eric Vivier, Lucas Rebuffet, Emilie Narni-Mancinelli, Stéphanie Cornen, Rob Y. Igarashi5 and Valeria R. Fantin5. " Natural killer cell therapies" Cell 626(2024): 727-736.
Acknowledgments
Produced from materials originally authored by Kun Shi, Na Zhang, Xing Zhang, Spencer Chiang, Haonan Li, and Tianfu Zhang from ACROBiosystems.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.