An important step in the production of CAR-T cells is the evaluation of CAR expression. Usually, this is performed by flow cytometry using target antigens, protein L, or anti-Fab antibodies as detection antibodies. Among these standard options, target antigens are broadly considered to be the right choice as it provides minimal background staining and high specificity.
| Reagents |
Mechanism |
Pros |
Cons |
| Target Antigens |
Specifically bind to the antigen-binding domains of CARs
|
High specificity
Minimal background staining
|
High cost
Each unique CAR has to be stained with corresponding antigens
|
| Protein L |
Binds to the kappa light chain of immunoglobulin
|
Universal and low cost
|
High background staining.
Cannot detect the anti-lambda light chain CAR
|
| Anti-Fab antibody |
Binds to the Fab portion of immunoglobulin
|
Universal and low cost
|
High background staining
|
Case Studies
Case No.1 Evaluation of Anti-BCMA CAR Expression Using Biotinylated BCMA
Reagents:
- PE Streptavidin (Biolegend, Cat. No. 405204)
- Biotinylated human BCMA protein, Fc & Avi Tag (ACROBiosystems, Cat. No. BC7-H82F0)
Samples:
Anti-BCMA CAR-transduced human primary T-cells
Brief Protocol:
- Human primary T-cells is traduced with a lentiviral vector for expressing anti-BCMA CAR
- Three days after transduction, the cells were stained with a biotinylated human BCMA protein (ACROBiosystems, Cat. No. BC7-H82F0)
- Secondary labeling was carried out with PE Streptavidin after washing
- The cells were examined with the help of BD FACSCaliburTM flow cytometer, and the data were analyzed using FCS Express 6 Plus software
Results:
The data demonstrated that the expression of anti-BCMA CARs on transduced T cell surface from donor 2 and donor 1 were 73.49% and 52.72%, respectively.
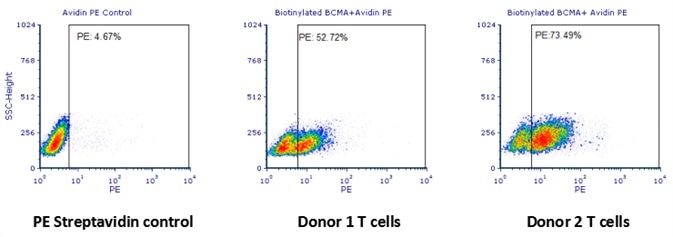
Human T cells were transfected with anti-BCMA CAR and cultured for 3 days. Three days post-transfection, 1x106 cells were first incubated with 50 μl biotinylated human BCMA protein (Cat. No. BC7-H82F0, 8 μg/ml), washed and then stained with PE Streptavidin and analyzed by flow cytometry. (Data are kindly provided by PREGENE Biopharma). Image credit: ACRO Biosystems
Case No.2 Evaluation of Anti-CD19 CAR Expression Using Biotinylated CD19
Reagents:
- FITC Streptavidin (Biolegend, Cat. No. 405201)
- Biotinylated human CD19 protein, Fc Tag (ACROBiosystems, Cat. No. CD9-H8259)
Samples:
R1013-C6 cells (Transfected 293 cells expressing the anti-CD19 [FCM63] scFv & RFP tag).
Brief Protocol:
- Expi 293 cells were transfected with a mammalian expression vector to steadily express anti-CD19 [FCM63] scFv & RFP tag. The resulting stable cell lines were labeled as R1013-C6 cells
- For CAR expression, cells were stained using a biotinylated human CD19 protein (ACROBiosystems, Cat. No. CD9-H8259)
- After washing, secondary labeling was carried out with FITC Streptavidin
- The cells were examined using NovoCyteTM flow cytometer and the data were analyzed by ACEA NovoExpress software
Results:
The data demonstrated that, in particular, the interaction of anti-CD19-CAR and CD19 mediated the binding of biotinylated human CD19 (Cat. No. CD9-H8259) to anti-CD19 scFv-modified cells.
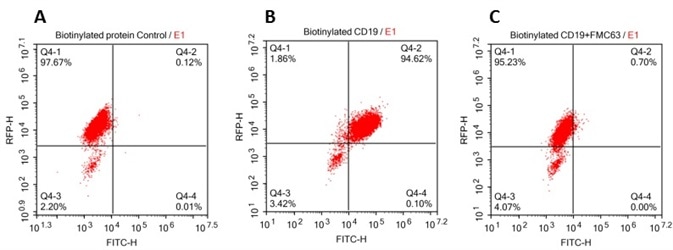
293 cells were transfected with FCM63-scFv and RFP tag. 2x105 of the cells were first incubated with A. Biotinylated protein control. B. Recombinant biotinylated human CD19 (Cat. No. CD9-H8259, 10 μg/ml). C. Recombinant biotinylated human CD19 (Cat. No. CD9-H8259, 10 μg/ml) and FMC63(Mouse anti-CD19 antibody). FITC Streptavidin was used to analyze with FACS. RFP was used to evaluate CAR(FMC63-scFv) expression and FITC was used to evaluate the binding activity of recombinant biotinylated human CD19 (Cat. No. CD9-H8259). Image credit: ACRO Biosystems
Case No.3 Evaluation of Anti-CD19 CAR Expression Using Biotinylated CD19
Reagents:
- Brilliant Violet 711™ anti-human CD3 Antibody (BioLegend, Cat. No.300463)
- PE Streptavidin (Biolegend, Cat. No.405204)
- Biotinylated human CD19 protein, Fc Tag (ACROBiosystems, Cat. No. CD9-H8259)
Samples:
TRC1-2-treated, AAV:TRAC:CAR-transduced T cells.
Brief Protocol:
- After the activated T cells were electroporated with TRC1-2 mRNA, they were transduced with AAV:TRAC:CAR at an MOI of 400,000 vg/cell and cultured for five days in the presence of IL-2
- Five days after transduction, cells were stained for the CAR expression using a biotinylated human CD19 protein (ACROBiosystems, Cat. No. CD9-H8259), and subsequently stained with PE Streptavidin and CD3 using a Brilliant Violet 711™ anti-human CD3 Antibody
- The cells were examined using BD Fortessa flow cytometer and the data were analyzed by FlowJo software
Results:
A high frequency of CD19 CAR+ cells in the CD3- population is demonstrated in the analysis of CD3 and CAR expression by flow cytometry.
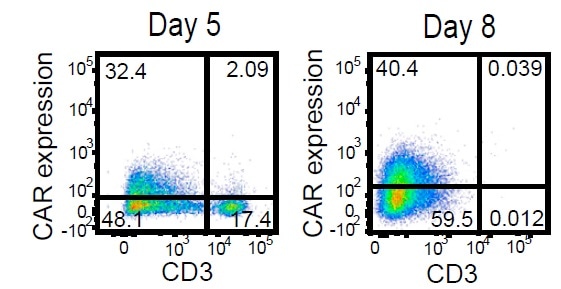
Activated T cells were electroporated with TRC1-2 mRNA and transduced with AAV:TRAC:CAR at an MOI of 400,000 vg/cell and cultured for 5 days in the presence of IL-2. Five days post-transduction, cells were stained for expression of the CAR using a biotinylated CD19-Fc reagent and CD3, with TRC1-2-treated, mock-transduced cells used as a control for gating of CAR expression. CD3+ cells were then depleted. Enriched CD3- cells were cultured for 3 additional days in the presence of IL-15 and IL-21 and then analyzed again by flow cytometry for CD3 and CAR expression. Image credit: ACRO Biosystems
Case No.4 Evaluation of Anti-CD19 CAR Expression Using CD19, His Tag
Reagents:
- FITC anti-6xHis tag antibody (Abcam, Cat. No. ab1206)
- Human CD19 protein, His Tag (ACROBiosystems, Cat. No. CD9-H52H2)
Samples:
R1013-C6 cells (Transfected 293 cells expressing the anti-CD19 [FCM63] scFv & RFP tag).
Brief Protocol:
- Expi 293 cells were transfected with a mammalian expression vector for a stable expression of anti-CD19 [FCM63] scFv & RFP tag. The resulting stable cell lines were labeled as R1013-C6 cells
- For CAR expression, cells were stained using a human CD19 protein, His Tag (ACROBiosystems, Cat. No. CD9-H52H2)
- Secondary labeling was carried out with FITC anti-6xHis tag antibody after washing
- The cells were examined using Accuri C6 flow cytometer and data analyzed GraphPad Prism 5 and FCS Express 6 Plus software
Results:
The data demonstrated that the binding of human CD19, His Tag (Cat. No. CD9-H52H2) to anti-CD19 scFv-modified cells was particularly mediated by the interaction of anti-CD19-CAR and CD19.
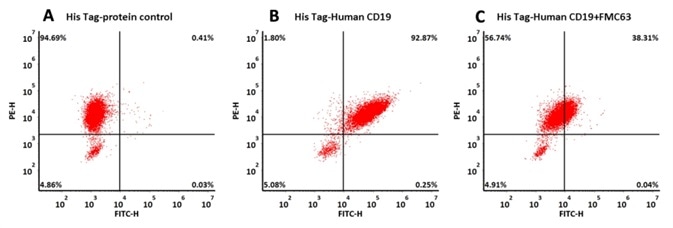
293 cells were transfected with FMC63-scFv and RFP tag . 2x105 of the cells were first incubated with A. His Tag-protein control. B. Recombinant human CD19, His Tag (Cat. No. CD9-H52H2, 10 μg/ml). C. Recombinant human CD19, His Tag (Cat. No. CD9-H52H2, 10 μg/ml) and FMC63 (Mouse anti-CD19 antibody). The FITC Anti-6xHis tag antibody was used to analyze with FACS. RFP was used to evaluate CAR (FMC63-scFv) expression and FITC was used to evaluate the binding activity of recombinant human CD19, His Tag (Cat. No. CD9-H52H2). Image credit: ACRO Biosystems
Case No.5 Evaluation of Anti-CD19 CAR Expression Using CD19, Fc Tag
Reagents:
- FITC anti-human IgG Fc antibody (Biolegend, Cat. No. 409310)
- Human CD19 protein, Fc Tag (Acrobiosystems, Cat. No. CD9-H5259)
Samples:
R1013-C6 cells (Transfected 293 cells expressing the anti-CD19 [FCM63] scFv & RFP tag).
Brief Protocol:
- Expi 293 cells were transfected with a mammalian expression vector for stably expressing anti-CD19 [FCM63] scFv & RFP tag. The ensuing stable cell lines were labeled as R1013-C6 cells
- Cells were stained for the expression of the CAR using a human CD19 protein, Fc Tag (ACROBiosystems, Cat. No. CD9-H5259) for CAR expression
- After washing, secondary labeling was carried out with FITC anti-human IgG Fc antibody
- The cells were examined using NovoCyteTM flow cytometer and the data were analyzed using ACEA NovoExpress software
Results:
The data demonstrated that the human CD19, Fc Tag (Cat. No. CD9-H5259) to anti-CD19 scFv-modified cells binding was particularly mediated by the interaction between anti-CD19-CAR and CD19.
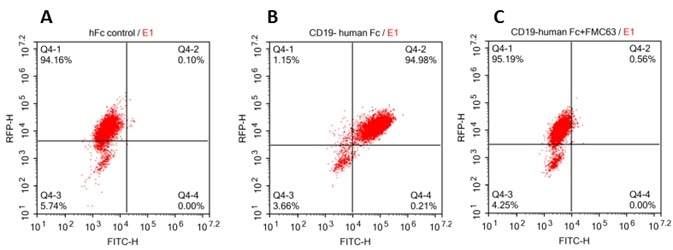
293 cells were transfected with FCM63-scFv and RFP tag . 2x105 of the cells were first incubated with A. Human Fc tag control. B. Recombinant human CD19, Fc Tag (Cat. No. CD9-H5259, 10 μg/ml). C. Recombinant human CD19, Fc Tag(Cat. No. CD9-H5259, 10 μg/ml) and FMC63(Mouse anti-CD19 antibody), followed by FITC anti-human IgG Fc antibody, and then analyzed using NovoCyteTM Flow Cytometer. RFP was used to evaluate CAR(FMC63-scFv) expression and FITC was used to evaluate the binding activity of recombinant human CD19, Fc Tag. Image credit: ACRO Biosystems
Case No.6 Evaluation of the Detection Specificity of CD19, Fc Tag
Reagents:
- Human CD19 Protein, Fc Tag (Company N)
- Human PD-L1 Protein, Fc Tag (ACROBiosystems, Cat. No. PD1-H5258), as negative control
- FITC anti-human IgG Fc antibody (Biolegend, Cat. No. 409310)
- Human CD19 Protein, Fc Tag (ACROBiosystems, Cat. No. CD9-H5259)
Cells:
R1013-C6 cells (Transfected 293 cells expressing the anti-CD19 [FCM63] scFv & RFP tag); Jurkat E6.1 cells, Expi 293 cells.
Brief Protocol:
- ACRO’s human CD19 protein, Company N’s human CD19 protein, or Fc Tag was used to stain the cells for CAR expression
- Secondary labeling was carried out with FITC anti-human IgG Fc antibody after washing
- The cells were examined using NovoCyteTM flow cytometer and the data were analyzed by FCS Express 6 Plus and GraphPad Prism 5 software
Results:
According to the data, Company N’s human CD19-Fc fusion protein exhibits strong non-specific binding to 293 and Jurkat cells.
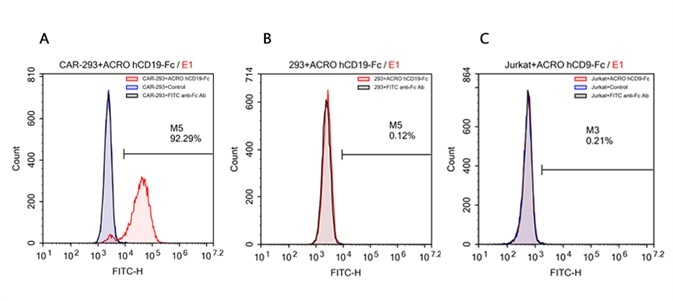
FACS analysis of human CD19 protein, Fc Tag (ACROBiosystems, Cat. No. CD9-H5259) binding to A. R1013-C6 cells, B. Expi 293 cells, C. Jurkat E6.1 cells. Cells were first stained with human CD19 protein, Fc Tag (ACROBiosystems, Cat. No. CD9-H5259) followed by FITC anti-human IgG Fc antibody, and then analyzed using NovoCyteTM Flow Cytometer. The data were analyzed with FCS Express 6Plus and GraphPad Prism 5 software. Image credit: ACRO Biosystems
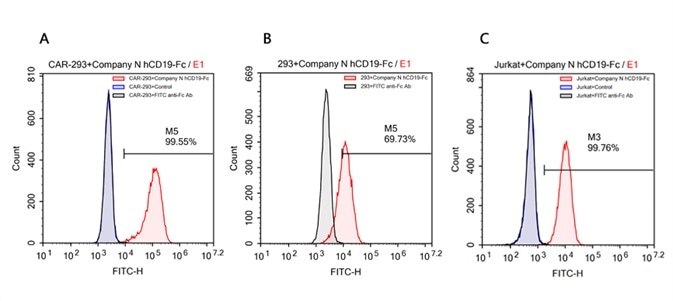
FACS analysis of human CD19 protein, Fc Tag (Company N) binding to A. R1013-C6 cells, B. Expi 293 cells, C. Jurkat E6.1 cells. Cells were first stained with human CD19 protein, Fc Tag (Company N) followed by FITC anti-human IgG Fc antibody, and then analyzed using NovoCyteTM Flow Cytometer. The data were analyzed with FCS Express 6Plus and GraphPad Prism 5 software. Image credit: ACRO Biosystems
Reference
- MacLeod DT, et al., 2017, Mol Ther. 25(4):949-961.doi: 10.1016/j.ymthe.2017.02.005.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.