High-density microelectrode arrays (HD-MEAs) play a crucial role in assessing the functional characteristics of cerebral organoids. As a pioneer in this area, 3Brain specializes in developing top-tier HD-MEAs, which facilitate accurate, high-resolution electrophysiological recordings.
ACROBiosystems, a worldwide biotechnology firm, produces and supplies both ready-to-use and cryopreserved human iPSC-derived cerebral organoids for research and drug development purposes. These organoids replicate essential features of human cerebral cortex structure and function, making them invaluable in vitro models for scientific inquiry.
This article examines the modulation of activity in ACROBiosystems’ human iPSC-derived cerebral organoids following chemical stimulation with Bicuculline, 4-AP, and TTX. Utilizing the BioCAM DupleX and CorePlate™ 1W 38/60, ACROBiosystems analyze the organoids' functional responses to these established pharmacological agents.
Introduction
Human iPSC-derived cerebral organoids serve as an effective in vitro model for exploring cerebral development, disease modeling, and neuropharmacological reactions.
These three-dimensional, self-organizing entities replicate important aspects of human cerebral cortex structure and function, providing insights into critical research fields such as development and neuropharmacology.
One of the key applications of brain organoids is evaluating acute electrophysiological responses to pharmacological agents.
This methodology provides valuable information regarding various neuronal properties, including excitability, receptor functionality, and network dynamics. Furthermore, it enables researchers to determine how closely organoid behavior aligns with in vivo brain activity, presenting a promising platform for the investigation of new neuropharmacological compounds.
Electrophysiological responses can be effectively characterized using high-density microelectrode arrays (HD-MEAs).
The BioCAM DupleX is an advanced HD-MEA system featuring integrated temperature regulation, a 20 kHz sampling rate, and 4,096 bidirectional recording electrodes.
This system allows for data collection from multiple CorePlate™ HD-MEAs, leveraging 3Brain's cutting-edge technology for high-resolution, label-free electrophysiological measurements with exceptional precision. This capability enables the capture of extensive data from complex biological samples like cerebral organoids, fostering deeper insights and enhancing research efforts.
ACROBiosystems is a global biotechnology leader capable of producing and supplying both ready-to-use and cryopreserved human iPSC cerebral organoids for research applications.
These organoids have undergone histological and immunohistochemical characterization by ACROBiosystems, confirming the presence of typical neuronal and glial cell markers, validated through immunostaining and single-cell RNA sequencing (Fig. 1).
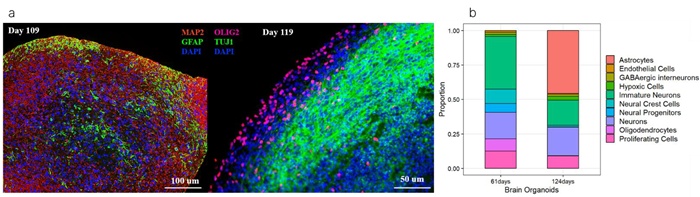
Figure 1. Immunostaining (a) and scRNA-seq analysis (b) showing that cerebral organoids highly express mature neurons and glia after 100+ days (CIPO-BWL002K, ACROBiosystems), compared to 60+ day organoids (CIPO-BWL001K, ACROBiosystems). Image Credit: ACROBiosystems
This article complements these findings by detailing their electrophysiological responses using the BioCAM DupleX & CorePlate™ 1W 38/60 (21 µm x 21 µm electrodes, 60 µm pitch, 3.8 mm2 recording area) to known neuropharmacological agents: 4-Aminopyridine (4-AP), a potassium channel blocker; Bicuculline (Bic), a GABA-A receptor antagonist; and Tetrodotoxin (TTX), a voltage-gated sodium channel blocker.
The team at ACROBiosystems will also illustrate how high-resolution electrophysiological data has been utilized to characterize ACROBiosystems' human iPSC-derived cerebral organoids, thereby enabling researchers to optimize experimental workflows and advance neuropharmacological research and drug discovery.
Methods
Cells, culture method & reagents
ACROBiosystems cultured organoids following their internal protocol,1 utilizing the RIPO-BWM001K kit for differentiating iPSC-derived cerebral organoids and the RIPO-BWM003 kit for the maintenance and maturation of these organoids.
The organoids were allowed to mature for 100 days (CIPO-BWL002K, ACROBiosystems) before being sent to 3Brain.2 After this, the organoids were cultured in BrainPhys™ (StemCell) for an additional 15 days prior to recording.
Dosing & stimulation protocol
The organoids underwent the following steps (illustrated in Fig. 2):
- A baseline recording lasting 3 minutes was performed.
- Organoids were then treated with 100 µM 4-AP and 20 µM Bic (Sigma) and recorded for another 3 minutes.
- Subsequently, organoids were administered 10 µM TTX (Sigma) and recorded for an additional 3 minutes.
HD-MEA
The BioCAM DupleX, along with CorePlate™ 38/60, was employed to capture extracellular electrophysiological activity from the organoids. Recorded data was analyzed using BrainWave 5.
Results
Spiking activity is altered by 4-AP, Bic and TTX
The application of 4-AP, bicuculline, and TTX resulted in notable changes in spiking activity, as illustrated in the raster plot in Fig. 3.
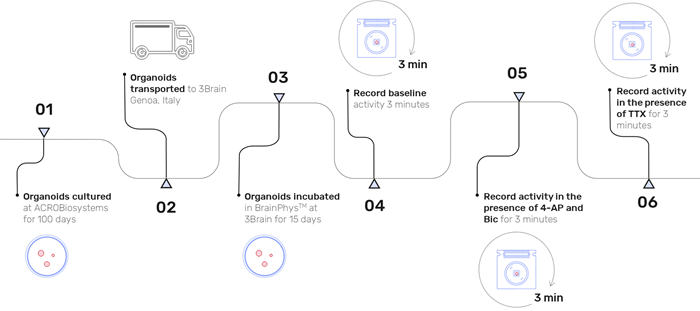
Figure 2. Experimental timeline illustrating the culture period, organoid delivery, organoid incubation in BrainPhys™ and three recording stages: baseline activity, activity after addition of 100 μM 4-AP + 20 μM Bic, activity after addition of 10 μM TTX. Image Credit: ACROBiosystems
After administering 4-AP and bicuculline, there was a reduction in spontaneous neuronal activity, while synchronized network activity increased, indicating diminished network inhibition. Conversely, the introduction of TTX caused a significant decline in activity, suggesting a suppression of neuronal excitability.
Network characteristics are altered by 4-AP, Bic and TTX
Following the addition of 4-AP and bicuculline, the frequency of network bursts increased, demonstrating enhanced excitability and decreased inhibition within the network. This effect was completely reversed with the application of TTX. The duration of network bursts significantly diminished with 4-AP and bicuculline and was completely eliminated with TTX.
Additionally, the interval between network bursts was shortened after the application of 4-AP and bicuculline, indicating more frequent bursting events. Similarly, the inter-spike interval during network bursts significantly decreased with 4-AP and bicuculline, further reflecting increased excitability, which was abolished by TTX (Fig. 4).
Network connectivity is altered by 4-AP and Bic
The level of network connectivity changed following the addition of 4-AP and bicuculline, evidenced by a significant rise in correlation values and node degree. This suggests that the compounds influenced the integrity of the network, likely due to reduced inhibition (Fig. 5).
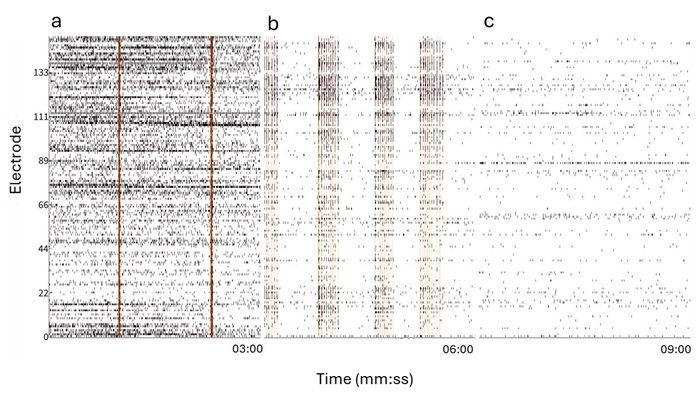
Figure 3. Raster Plot displaying the spiking activity of the organoid from a subset of example electrodes at baseline (a), 100 μM 4-AP + 20 μM Bic (b), 10 μM TTX (c). Orange bars indicate network burst detection. Image Credit: ACROBiosystems
The connectivity map overlaid on the organoid in Fig. 6 visually represents these alterations, showcasing an increased number of links, their distribution, and correlation strength.
Centre of activity trajectory is modulated by 4-AP and Bic
The Centre of Activity Trajectory (CAT) illustrates the dynamic movement of network bursts over time, capturing the spatial propagation of neural activation across the sample.3
CAT analysis revealed a significant increase in velocity and a decrease in duration following the addition of 4-AP and bicuculline (Fig. 7), likely due to increased neuronal excitability from potassium channel blockade and reduced GABAergic inhibition. These changes are depicted in the CAT map (Fig. 8), where the expanded trajectory distribution across the organoid is apparent.
Discussion
In vitro models of the cerebral cortex are essential for enhancing our understanding of cerebral development and function, as well as for advancing drug discovery and neuropharmacological research.
ACROBiosystems offers both ready-to-use and cryopreserved human iPSC-derived cerebral organoids for research and drug development, which can be shipped globally to provide a standardized in vitro model for critical neuroscientific studies and drug response assessments.
This research utilized the BioCAM DupleX and CorePlate™ 1W 38/60 to gather high-resolution electrophysiological data for characterizing an ACROBiosystems human iPSC-derived cerebral organoid.
The electrophysiological recordings provided valuable insights into the dynamics of the organoid’s neural network. The observed changes in spiking activity after bicuculline application suggest that the organoids contain functional inhibitory neurons that actively regulate network activity.
By decreasing inhibition, bicuculline, a GABA-A receptor antagonist, facilitated synchronized network-wide activity. The concurrent application of 4-AP, a potassium channel blocker, likely further enhanced network synchrony by boosting excitability. In contrast, TTX, a sodium channel blocker, diminished network activity.
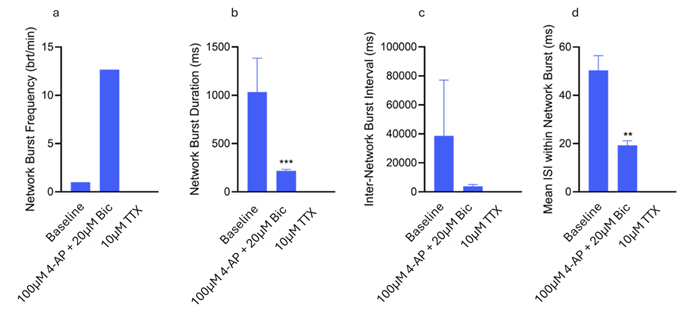
Figure 4. Network Burst metrics displaying the average Network Burst Frequency (a), Network Burst Duration (b), Inter Network Burst Interval (c) and Inter-Spike Interval within Network Bursts (d). Significant differences in Network Burst Duration and Mean ISI within Network bursts were found between Baseline & 100 μM 4-AP + 20 μM Bic (p=0.0008 & p=0.0019 respectively) (Kolmogorov-Smirnov test). Image Credit: ACROBiosystems
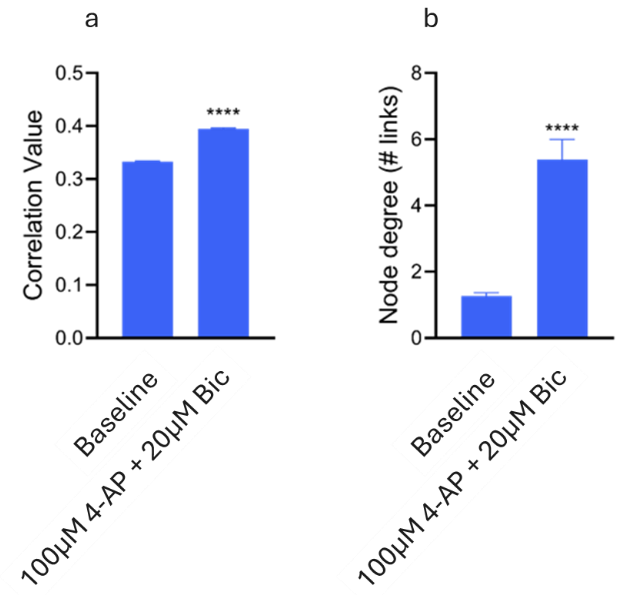
Figure 5. Connectivity metrics displaying the correlation value (a) and node degree (# links) (b). Significant differences in correlation value and node degree were found between Baseline & 100 μM 4-AP + 20 μM Bic (p<0.0001) (Kolmogorov-Smirnov test). Image Credit: ACROBiosystems
The alterations in network bursting properties observed following bicuculline, 4-AP, and TTX treatment further confirm the presence of a functional network within the organoids. The increased burst frequency and decreased inter-burst intervals associated with 4-AP and bicuculline likely stem from disinhibition and heightened excitability.
Additionally, the reduction in burst duration and inter-spike intervals within bursts indicates a transition toward shorter, more frequent network events, typical of reduced inhibition and a hyperexcitable state. This increased activity was effectively suppressed by TTX.
Using BrainWave software, the network connectivity within the cerebral organoids was analyzed with ease. The rise in correlation and link numbers following the addition of 4-AP and bicuculline further emphasizes the effects of decreased inhibition and increased excitability on the organoid network.
The visualization of this connectivity map overlaid on the organoid (Fig. 6) illustrates these changes, revealing a greater number of connections and a wider distribution of nodes.
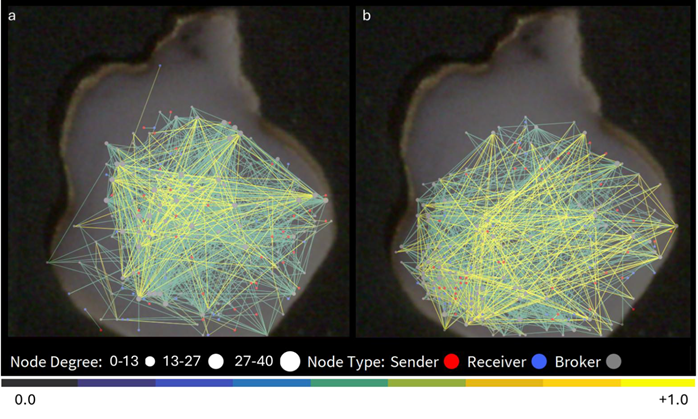
Figure 6. Network connectivity map overlayed on the recorded organoid in baseline condition (a), and after application of 4- AP and Bic (b). Image Credit: ACROBiosystems
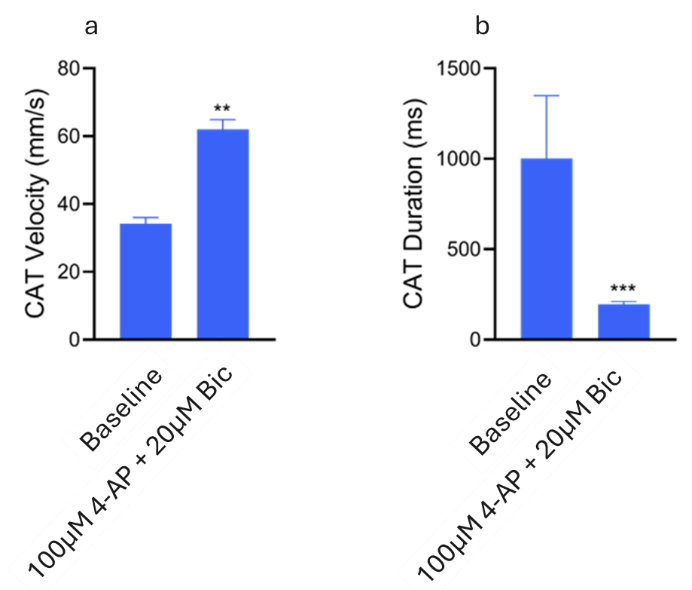
Figure 7. CAT metrics displaying the CAT velocity (a) and CAT duration (b). Significant differences in CAT velocity and CAT duration were found between Baseline & 100 μM 4-AP + 20 μM Bic (p=0.0049 & p=0.0008 respectively) (Kolmogorov-Smirnov test). Image Credit: ACROBiosystems
Finally, alterations in the CAT were observed, with an increase in CAT velocity and a reduction in duration following treatment with 4-AP and bicuculline (Fig. 7), indicating rapid changes in network activity and reflecting a more excitable network with less inhibition.
The expanded CAT trajectory distribution was visualized as an overlay on the organoid (Fig. 8), suggesting that reduced inhibition allows for broader activity.
Overall, these findings demonstrate that pharmacological modulation with 4-AP, bicuculline, and TTX influences neuronal activity and network characteristics within the human iPSC-derived cerebral organoid (CIPO BWL002K, ACROBiosystems) in a predictable manner following the overseas shipment of live organoids.
The BioCAM DupleX and CorePlate™ 38/60 (3Brain) enabled precise, high-resolution analysis of these functional changes, providing insights into the neuronal functionality and network properties of the organoids. This underscores the potential of CorePlate™ in advancing research on the characterization of in vitro cerebral organoid models.
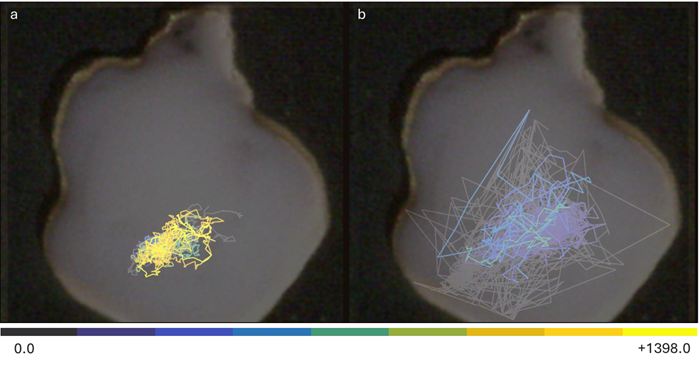
Figure 8. CAT Map overlayed on the recorded organoid displaying trajectory in baseline condition (a), and after application of 4-AP and Bic (b). Image Credit: ACROBiosystems
References
- Acrobiosystems. (2017). Human iPSC-Derived Cerebral Organoid Differentiation Kit - ACROBiosystems. (online) Available at: https://www.acrobiosystems.com/P7373-Human-iPSC-Derived-Cerebral-Organoid-Differentiation-Kit.html (Accessed 19 May 2025).
- Acrobiosystems. (2017). Ready-to-use Human iPSC-Derived Cerebral Organoids - ACROBiosystems.(online)Available at: https://www.acrobiosystems.com/P7372-Ready-to-use-Human-iPSC-Derived-Cerebral-Organoids.html (Accessed 19 May 2025).
- Gandolfo, M., Alessandro Maccione, Tedesco, M., Martinoia, S. and Luca Berdondini (2010). Tracking burst patterns in hippocampal cultures with high-density CMOS-MEAs. 7(5), pp.056001–056001. https://doi.org/10.1088/1741-2560/7/5/056001.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.